Machine learning predicts the look of stem cells – Nature.com
By raymumme
No two stem cells are identical, even if they are genetic clones. This stunning diversity is revealed today in an enormous publicly available online catalogue of 3D stem cell images. The visuals were produced using deep learning analyses and cell lines altered with the gene-editing tool CRISPR. And soon the portal will allow researchers to predict variations in cell layouts that may foreshadow cancer and other diseases.
The Allen Cell Explorer, produced by the Allen Institute for Cell Science in Seattle, Washington, includes a growing library of more than 6,000 pictures of induced pluripotent stem cells (iPS) key components of which glow thanks to fluorescent markers that highlight specific genes.
The Cell Explorer complements ongoing projects by several groups that chart the uniqueness of single cells at the level of DNA, RNA and proteins. Rick Horwitz, director of the Allen Institute for Cell Science, says that the institutes images may hasten progress in stem cell research, cancer research and drug development by revealing unexpected aspects of cellular structure. You cant predict the outcome of a football game if you know stats on all the players but have never watched a game.
The project began about a year ago with adult skin cells that had been reprogrammed into an embryonic-like, undifferentiated state. Horwitz and his team then used CRISPRCas9 to insert tags in genes to make structures within the cells glow. The genes included those that code for proteins that highlight actin filaments, which help cells to move and maintain their shape. It quickly became clear that the cells, which were all genetic clones from the same parent cell, varied in the placement, shape and number of their components, such as mitochondria and actin fibres.
Computer scientists analysed thousands of the images using deep learning programs and found relationships between the locations of cellular structures. They then used that information to predict where the structures might be when the program was given just a couple of clues, such as the position of the nucleus. The program learned by comparing its predictions to actual cells.
The deep learning algorithms are similar to those that companies use to predict peoples preferences, Horwitz says. If you buy a chainsaw at Amazon, it might then show you chain oil and plaid shirts.
The 3D interactive tool based on this deep learning capability should go live later this year. At the moment, the site shows a preview of how it will work using side-by-side comparisons of predicted and actual images.
Benjamin Freedman, a cell biologist at the University of Washington in Seattle, looks forward to playing with the Cell Explorers predictive function once the Allen Institute team has taught their algorithm to recognize more iPS cells that have been changed genetically or chemically. For example, Freedman says he could delete a gene related to kidney disease in one of the fluorescently tagged stem cells from the Allen Institute and see how the mutation affects the glowing structure. Then he could use the sites modelling tool to determine how other cellular components might be altered. Ultimately, Freedman says, we want to understand processes at the cellular level that cause disease in the kidney as a whole.
In the coming months, Allen Institute researchers will update the site with images of stem cells at different stages of cell division, and as they transform into distinct cell types, such as heart and kidney cells. Catching cells at different time points can be crucial to identifying fundamental processes, says Horwitz.
Allen Institute for Cell Science
Structural differences in the DNA (purple) and cellular membrane (blue) of genetically identical stem cells.
The Allen Institutes visual emphasis on stem cells dovetails with a number of efforts to catalogue other aspects of cells. For example, the London-based charity Cancer Research UK is creating interactive virtual-reality models of breast cancer cells in tumours. And an international effort called the Human Cell Atlas seeks to define all human cell types in terms of their molecular profiles, including DNA sequences, RNA transcripts and proteins.
Aviv Regev, a computational biologist at the Broad Institute in Cambridge, Massachusetts, who is working on the Human Cell Atlas, says that the Allen Cell Explorer complements her project by focusing on the look of cellular features as opposed to how genes, RNA and proteins interact within the cell. The community is accepting that there are a lot of differences between cells that we thought were the same until recently, she says, so now were taking an unbiased approach to learn about pieces in the puzzle we didnt know existed before.
Originally posted here:
Machine learning predicts the look of stem cells - Nature.com
Man Receives Reprogrammed" Stem Cells From Donor In Medical First – IFLScience
By raymumme
Last week, a patient with blurry vision in his right eye walked into a doctors office and became the first person to receive reprogrammed stem cells from a donor to treat his age-related macular degeneration.
The patient a Japanese man in his 60s is not alone, as four other patients have been approved for the procedure by Japan's health ministry. The first medical case was reported on March 28 by Nature.
In a one-hour operation by surgeon Yasuo Kurimoto, the patient received skin cells from a human donor at Kobe City Medical Center General Hospital. The donors skin cells were reverse engineered into induced pluripotent stem (iPS) cells. These cells are often seen as a game-changer in the world of regenerative medicine as they have the ability to become almost any type of cell in the body.
In this case, the iPS cells were turned into retinal cells, which were then implanted into the retina of the patient, who has age-related macular degeneration. It is hoped the procedure will stop the progression of the disease, which can lead to blindness. The transplantis not being touted as a cure for the condition, merely a prevention methodfrom further damage.
During the procedure, the surgical team injected 50 microliters of liquid containing 250,000 retinal cells into the patients eye, according to the Japan Times. The real test, however, will be the next phase of monitoring.
What sets this transplant apart is also what makes the recovery process precarious. Doctors will need to keep a careful watch on the patient, as iPS cells from a donor are not a genetic match and could cause an immune rejection.
At this point, you might remember a similar case in 2014 with a Japanese woman at the same hospital. She also received retinal cells derived from iPS cells, however hers were taken from her own skin, not a donor's.
"A key challenge in this case is to control rejection," said Riken researcher Masayo Takahashi to the Japan Times. "We need to carefully continue treatment."
In an update, the team said the Japanese woman was doing well and her vision had not declined. They decided to change track and use donor cells for this study because it holds a more viable future for such transplants.
It's hoped, if all goes well here, that researchers can create a bank of donor stem cells. Such a future would cut down on costs and reduce wait times, as cultivating ones own cells can take several months. However, there's stillmuch to be done.
After the procedure, Takahashi told a press conference that the surgery went well. They will continue to monitor the situation and provide further updates in the future.
Read more:
Man Receives Reprogrammed" Stem Cells From Donor In Medical First - IFLScience
Benefit dinner will help family of baby girl recovering from bone marrow transplant – CTV News
By raymumme
A benefit dinner will be held April 1 in honour of seven-month-old Madalayna Ducharme. The Warrior Princess fundraiser starts Saturday at 5 p.m. at the Parkwood Gospel Temple. All proceeds will support the family's ongoing expenses related to her medical treatment.
Madalayna suffers from malignant infantile osteoporosis, a rare genetic disorder of bone development in which the bones become thickened and unhealthy. It leads to bone fractures, short stature, poor bone growth and a thicker skull which may delay development of teeth. Left untreated, it could be fatal.
Early this year, her family started a Facebook campaign that went viral asking for people to sign up to become stem cell or bone marrow donors. Thanks to the number of people who volunteered to be tested, a match was found and Madalayna underwent a bone marrow transplant earlier this month.
The recovery is expected to be lengthy as the transplant process is grueling on an infants body. Its expected that she will need to stay in a Toronto hospital for three months while she undergoes treatment.
Excerpt from:
Benefit dinner will help family of baby girl recovering from bone marrow transplant - CTV News
Donating the umbilical cord could save someone’s life – WNDU-TV
By raymumme
After a baby is born and the umbilical cord is cut, ever wonder where that umbilical cord ends up?
Most of the time, it becomes waste but that cord still has some valuable resources that can save a life.
The blood that is found in it is called umbilical cord blood or cord blood for short.
It contains all the normal elements of blood, such as red and white blood cells. It is also jam packed with stem cells, similar to the ones found in bone marrow.
Birth is pretty exciting, its pretty dramatic. A lot of things are happening, says James E. Baumgartner, M.D., Pediatric Surgeon.
One of those things that people rarely hear about is the option to donate cord blood. Bone marrow and cord blood contain the same type of stem cells, but those from cord blood have more advantages. Since stem cells from cord blood are less mature than stem cells from an adult's bone marrow, a recipient's body is less likely to reject them.
Another benefit is that taking cord blood is less invasive than a bone marrow transplant. Once an umbilical cord is clamped, it is wiped with antiseptic and a needle is inserted into one of the veins to withdraw a few ounces of blood. The procedure takes just a few minutes and is painless.
We all collect prospective data to look for risk for, you know, lung damage, kidney damage, liver damage, heart damage. Were looking at the nervous system pretty carefully and we found nothing. So that we really believe that its safe, explains Baumgartner.
About 70% of patients who need a stem cell transplant dont have a matching donor in their own family, which leads to the main advantage of cord blood. Stem cells from cord blood dont need to be exactly matched to the patient like bone marrow transplants from adult donors. One drawback to cord blood though is that the number of stem cells available is relatively small. This means young children will benefit because they need less.
Families can either save cord blood for themselves or donate it to a bank.
You need to talk to your doctor at least three months before your due date to find out if you are eligible to donate cord blood.
CORD BLOOD TREATMENT SAVES LIVES REPORT #2401
BACKGROUND: A stem cell transplant is a treatment that is used to treat cancers that affect blood and immune system like leukemia, multiple myeloma, and some types of lymphoma. Stem cell transplants are used to treat these types of cancer since the stem cells that the body naturally produces most often die due to treatments like radiation and chemotherapy. Human beings need stem cells to survive, therefore, a stem cell transplant gives patients blood cells that they cant produce anymore. Furthermore, donated cells can often find and kill the cancerous cells better than the patients own cells. Stem cells include:
* Red blood cells (RBCs) * White Blood cells (WBCs) * Platelets (Source: http://www.cancer.net/navigating-cancer-care/how-cancer-treated/bone-marrowstem-cell-transplantation/what-stem-cell-transplant-bone-marrow-transplant & https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/stem-cell-transplant/why-stem-cell-transplants-are-used.html)
CORD BLOOD: In the past, the only location where stem cells could be taken for a transplant was in the bone marrow. In recent years cord blood, the blood that is found in the umbilical cord, has been used for stem cell transplants. They possess the same quantity of stem cells as the bone marrow, and they come with more advantages. To start off, no surgery is needed like with bone marrow. Since the umbilical cord is natural in every birth, the mother can choose to donate her cord around three months before she is due. Once the cord is clamped, it is cleaned with antiseptic. Later, a needle is inserted into one of the veins in order to gather the necessary blood. Furthermore, since the cord blood stem cells are less mature than those stem cells from an adults bone marrow, the recipients body is less likely to reject the transplant. This is very important for people with ethnic backgrounds. With bone marrows stem cells, the match between the donor and the recipient has to be 8/8; with cord blood cells, on the other hand, the match can be partial. For recipients that come from an ethnic background, a perfect match can be harder to find. (Source: http://www.nationalcordbloodprogram.org/qa/what_are_advantages.html)
PROS & CONS: Other advantages that come with core blood cells are the association of lower incidence of GvHD (Graft vs. Host Disease), and the lower risk of viral infections. Nevertheless, the cord blood cells have a drawback: the amount of stem cells found in them is very small. Because of the low number, children benefit from this transplant procedure more than adults. Since childrens bodies are smaller, they need fewer cells for their body to start reproducing them naturally. On the other hand, adults naturally need more cells than the ones the cord blood produces because of their size. (Source: http://www.nationalcordbloodprogram.org/qa/how_is_it_collected.html)
See the article here:
Donating the umbilical cord could save someone's life - WNDU-TV
Maxwell Airman donates to stranger in need – Maxwell-Gunter Air Force Base
By raymumme
MAXWELL AIR FORCE BASE, Ala. --
With an upcoming permanent change of station, Maj. Mathew Carter, a Jeanne M. Holm Center for Officer Accessions and Citizen Development instructor at Air University, had hundreds of reasons to say no when he was asked to travel to Washington, D.C., to donate bone marrow to a complete stranger. Instead, he decided this opportunity was too important to loose.
Over the weekend of March 25, he underwent a bone marrow extraction procedure in the hope of helping a 7-year-old child he never met.
This story begins in 2003 when he registered with the Department of Defenses Salute to Life program.
Salute to Life, also known as the C.W. Bill Young Department of Defense Marrow Donor Recruitment and Research Program, was initiated in 1991 and is tailored to work exclusively with military members. Over time, the program has recruited more than 1 million donors.
Carter had registered for the program while his father was in the Army. His father was stationed at Ft. Sam Houston, Texas. Carter and his family attended a bone marrow drive held for their neighbor who was diagnosed with cancer.
After 14 years, he received not only a letter, but an email and a voicemail from the organization informing him of a match.
I was kind of caught off-guard by it. It was one of those things that had a lot of opportunities for me to say no, but I have a 5-year-old, and when they told me there was a child that has a very serious life-threatening disease, there was really no question. It was the right thing to do, so I said yes, said Carter.
Carter began the process in early February by being tested again to confirm the match and getting a physical. By late March he was ready for the procedure.
During the bone marrow extraction, the patient is under local anesthesia while the doctor uses a needle to remove the marrow from the back of the pelvic bone.
Initially before [the surgery] started I was a little anxious. Before you go into any surgery you get a little anxiety, but it was one of those things that I was ready to just do, he said. Afterward, it was just relief knowing that I had done all that I could possibly do to help this person out.
He compared bone marrow donation to other bodily donations in the sense that when you donate other organs, they are permanently removed. However, with bone marrow or stem cells, the body regenerates what is lost.
For the two to four weeks of being sore and tired after the procedure, you look at what the recipient is going through, and it pales in comparison, so having the opportunity to do something like that is just amazing, he said.
For the donor and the recipients safety, they are left completely anonymous.. Once a year has passed after the surgery, they are then given the choice to reach out to each other.
When asked what he would say to the child, he thought for a moment and said, Live your life to the fullest.
Carter hopes that through this experience he can help raise awareness about bone marrow and stem cell donation, and encourages other to sign-up as donors.
Youll be a little sore and tired, but have the opportunity to do something amazing, he said.
For more information about bone marrow or stem cell donation through Salute to Life, visit http://www.salutetolife.org.
Follow this link:
Maxwell Airman donates to stranger in need - Maxwell-Gunter Air Force Base
Japanese Man Is First to Receive "Reprogrammed" Stem Cells from Another Person – Scientific American
By raymumme
On March 28, a Japanese man in his 60s became the first person to receive cells derived from induced pluripotent stem (iPS) cells that had been donated by another person.
The surgery is expected to set the path for more applications of iPS cell technology, which offers the versatility of embryonic stem cells without the latters ethical taint. Banks of iPS cells from diverse donors could make stem cell transplants more convenient to perform, while slashing costs.
iPS cells are created by removing mature cells from an individual (from their skin, for example), reprogramming these cells backto an embryonic state, and then coaxing them to become a cell type useful for treating a disease.
In the recent procedure, performed on a man from Hyogo prefecture, skin cells from an anonymous donor were reprogrammed and then turned into a type of retinal cell that was transplanted onto the retina of thepatient who suffers from age-related macular degeneration. Doctors hope the cells will stop progression of the disease, which can lead to blindness.
In a procedure performed in September 2014at the Kobe City Medical Center General Hospital, a Japanese woman received retinal cells derived from iPS cells. They were taken from her own skin, though, and then reprogrammed. Such cells prepared for a second patient were found to contain genetic abnormalities and never implanted.
The team decided to redesign the study based on new regulations, and no other participants were recruited to the clinical study. In February 2017, the team reported that the one patient had fared well. The introduced cells remained intactand vision had not declined as would usually be expected with macular degeneration.
In todays procedure performed at the same hospital and by the same surgeon Yasuo Kurimoto doctors used iPS cells that had been taken from a donors skin cells, reprogrammed and banked. Japans health ministry approved the study, which plansto enroll 5 patients, on 1 February.
Using a donor's iPS cells does not offer an exact genetic match, raising the prospect of immune rejection. But Shinya Yamanaka, the Nobel Prize-winning stem-cell scientist who pioneered iPS cells, has contended that banked cells should be a close enough match for most applications.
Yamanaka is establishing an iPS cell bank, which depends on matching donors to recipients via three genes that code for human leukocyte antigens (HLAs) proteins on the cell surface that are involved in triggering immune reactions. HisiPS Cell Stock for Regenerative Medicine currently has cell lines from just one donor. But by March 2018, they hope to create 5-10 HLA-characterized iPS cell lines, which should match 30%-50% of Japans population.
Use of these ready-made cells has advantages for offering stem cell transplants across an entire population, says Masayo Takahashi, an ophthalmologist at the RIKEN Center for Developmental Biology who devised the iPS cell protocol deployed in todays transplant. The cells are available immediately versus several months wait for a patients own cells and are much cheaper. Cells from patients, who tend to be elderly, might have also accumulated genetic defects that could increase the risk of the procedure.
At a press conference after the procedure, Takahashi said the surgery had gone well but that success could not be declaredwithout monitoring the fate of the introduced cells. She plans to make no further announcements about patient progress until all five procedures are finished. We are at the beginning, she says.
This article is reproduced with permission and wasfirst publishedon March 28, 2017.
Here is the original post:
Japanese Man Is First to Receive "Reprogrammed" Stem Cells from Another Person - Scientific American
What are mesenchymal stem cells? – Palm Beach Post
By raymumme
In the United States alone, more than 400,000 lumbar discectomies and 500,000 spinal fusions are performed each year for symptoms related to lumbar disc degeneration. The ability to get these to heal without surgery has been a long-term goal of many patients and physicians alike. The Spine Center continues to be on the forefront of treatment options and is proud to offer stem cell therapy treatments for patients as part of our comprehensive non-operative treatment options.
Adult stem cells are divided into different categories. For example, the types of adult stem cells Dr. Theofilos uses to treat musculoskeletal issues are known as mesenchymal stem cells (MSCs). These are multi-potent cells that can differentiate into bone cells, cartilage cells, or fat cells.
The human body has multiple storage sites for stem cells to repair degenerated and injured structures. Dr. Theofilos has found that obtaining stem cells from the hip bone (iliac bone) is easily performed within minutes and, in most cases, is a fairly painless procedure for the patient. The stem cells are obtained from bone marrow; just minutes later, they are used for treatment.
This procedure is done in our office and after the procedure, the syringe of stem cells is taken to the lab and placed in a specialized machine called a centrifuge. The centrifuge spins the bone marrow solution and stem cells are separated from the non-useful cells. Now, the stem cells are ready for the treatment.
For those whom are ideal candidates, this provides great hope with reduction in pain and improved quality of life without the need for major surgery.
Voted as one of Americas Top Surgeons, Charles S. Theofilos, MD, Neurosurgeon and Founder of The Spine Center is a leading provider of the state-of-the-art, most comfortable and effective surgical, minimally invasive and non-surgical treatment options for a full range of cervical and spinal ailments, including stem cell therapy and artificial disc replacement. He was among a field of 20 top neuro and orthopedic surgeons in the U.S. chosen to participate in the groundbreaking Artificial Disc Study, which compared the clinical outcome of disc replacement versus traditional spinal fusion. A widely sought after educator and lecturer, Dr. Theofilos has offices in Palm Beach Gardens and Port St. Lucie. In an effort to maintain and honor the commitment to our patients, we will continue to accept Medicare and Medicare Advantage insurance plans for all new and follow up appointments.
11621 Kew Gardens Ave., Suite 101;Palm Beach Gardens
More here:
What are mesenchymal stem cells? - Palm Beach Post
‘Nigeria should harness potentials of regenerative medicine’ – Daily Trust
By raymumme
Dr. David Ikudayisi is the Medical Director, Glory Wellness & Regenerative Centre, a multi-specialty health care center, in Lagos and Abuja. Ikudayisi who is based in the United States said he is excited about the prospects of coming back home to help put Nigeria on the continental and global medical map through regenerative medicine. He also spoke on the controversy surrounding embroyonic stem cell therapy among others.
What is regenerative medicine allabout?
Regenerative Medicine is a branch of medicine that aims to restore normal function by repairing or replacing damaged or malfunctioning cells and tissues in patients who have lost tissue or organ function due to age, disease, or congenital defects. It comprises different components including, Platelet Rich Plasma (PRP) Therapy and Adult Stem Cell (ASC) Therapy, etc.
I was inspired into this branch of medicine when I was looking for alternative ways to alleviate my patients pain in USA without using addictive pain medications and frequent steroid injections. I discovered the benefits of the Platelet Rich Plasma and/or Adult Stem Cell Therapy for patients who were in need of joint pain relief, youthful appearance, or a restored sexual function.
In medical school, we were taught that the central nervous system rarely regenerates, that there is little or no hope for paralyzed patients, and that damaged brain tissue may be a permanent condition, just to name a few.
Nowadays, the re-growth of brain cells and improvements of neurological function in spinal cord injured patients have been documented. When applicable, adult stem cell treatment is basically a medical time machine. The results doctors see in medical practice every day is what keeps my drive for the advancement of regenerative medicine.
How will regenerative medicine help address health care challenges in Nigeria?
Nigeria has a lot of ways to go when it comes to health, as we are recording very poor health indices in recent times. This can be traced to not enough emphasizes on preventive medicine or regenerative medicine. We tend to lose hope at different stages of degrading health rather than come up with solutions that can be attainable no matter the initial cost. If taken without levity, Regenerative Medicine, as in Platelet Rich Plasma (PRP) Therapy & Adult Stem Cell (ASC) Therapy, can help reduce our mortality rate in Nigeria, thereby recording better health indices.
This branch of medicine holds answers to many questions and problems that we doctors used to believe had no solutions. Many medical conditions that we thought were not treatable are now treatable. We have ample evidence to show the potentials of regenerative medicines for ailments that have domineered our people. The use of these techniques will propel Nigerian healthcare to boundless heights if provided the opportunity.
Regenerative medicine has a vast amount of uses especially for people who seek to stop being dependent on taking medications daily, avoid surgery, feel younger and more energized, perform their marital enjoyment at older ages, and prevent the manifestation of some complications of diseases.
Many countries around the world are taking advantage of these therapies, and Nigeria should not be left behind. I know this is not an undertaking with quick payoff nationally, but I believe that with these innovations, Nigeria can be the centre for medical tourism in Africa. This is not a cheap option, as most novel innovations never are, but it will pay dividends in the long run, and we have seen proofs of that.
Some doctors are already offering regenerative medicine (PRP therapy and ASCT) in Nigeria. So, people dont have to travel abroad to benefit from regenerative medicine. Just ask your doctor if someone in your area is offering ASCT and PRP Therapy.
What illnesses does ASCT and PRP therapy treat?
The applications of ASCT are enormous, and there is much more to be discovered. To determine if this can be of use in a particular medical problem, just ask if there is a need for repair and/regeneration of any part of the human tissue/organ: if yes, then the ASCT with/without PRP therapy may be an option that will help. The exceptions are non-hematological cancer treatments, as these treatments with stem cells are under investigations/research using tissue engineering.
More specifically, these therapies can be used for issues like multiple joint pain, back pain, meniscal tears, ligament tears, avascular necrosis of the hip joints, facet arthropathy, hair thinning, erectile dysfunction, female sexual dysfunction, female urinary incontinence, cosmetic/aesthetic applications (vampire facial, vampire facelift, vampire breast and nipple lift); diabetes, hypertension, anti-aging (generalized treatment), chronic kidney disease, multiple sclerosis, cerebral palsy, spinal cord injury, COPD (lung disease) and infertility (helps to increase fertility chances and not serve as a cure), to mention a few.
Why is there controversy around embryonic stem cell therapy?
The ethical, religious and moral arguments for and against Embryonic Stem Cell use has been stressed for many years now. Stem cells possess the ability to make new cells needed in the body. Embryonic stem cells are an example of this. In the body, they are what we use to develop the cells we later go on to have.
Many scientists prefer it because of its endless possibilities to recreate virtually any type of cell in the body. However, it involves the use of embryos from day 6 to day 14; that is, a baby-to-be in the first two weeks of pregnancy or after In vitro fertilization (IVF). As you can see, this involves tampering with potential or future babies that are yet to be born. This has both religious and ethical issues, leading to the controversy around it.
What is the difference between adult stem cell therapy and cloning?
It is important to understand that there are three main types of stem therapies. In addition to Embryonic Stem Cell (ESC) therapy, there are also induced Pluripotent Stem Cells (iPSCs) therapy and Adult Stem Cells (ASC)therapy. iPSCs are produced in the lab by reprogramming adult cells to express embryonic stem cells characteristics, and Adult Stem Cells (ASC) are retrieved from individuals bone marrow, adipose (fat) or umbilical cord. Although ASCs can be used for a vast number of therapies, they do not possess the same capabilities as ESCs, but the downside of limited growth and differentiation makes ASCs applicable in medicine today.
Cloning on the other hand is the process of producing similar populations of genetically identical organisms to sometimes replace damaged or lost tissues or organs. Every single bit of their DNA is identical to the original specimen. Clones can happen naturally, like identical twins or triplets, or they can be made in the lab, like Dolly the sheep. Reproductive cloning for humans has been banned in several countries, and mainstream scientists consider it unethical.
Link:
'Nigeria should harness potentials of regenerative medicine' - Daily Trust
Unproven Stem Cell Treatment Blinds 3 Florida Women – ClickLancashire
By raymumme
The Food and Drug Administration recommends that people who consider getting a stem cell treatment need to make sure it has been approved or is being studied in a clinical trial that federal health regulators have allowed. The kind that have generated the most excitement - and controversy - are human embryonic stem cells, which are derived from early human embryos and can be coaxed to become any kind of cell in the body. After 20 years of research, Italian scientists recently received European regulatory approval for a stem cell-based treatment for a type of blindness that results from damage to the cornea, the surface of the eye. The fat tissue was then processed with enzymes with the goal of obtaining stem cells.
However, they immediately suffered complications, including retinal detachment and hemorrhage, which caused total loss of eyesight. "It's just not the case".
None of the women are named in the study.
In 2013, the company listed a trial on ClinicalTrials.gov titled, "Study to Assess the Safety and Effects of Cells Injected Intravitreal in Dry Macular Degeneration", according to the ClinicalTrials.gov site. Both lawsuits were settled, with the women receiving payments.
He warned that patients could be misled by "rogue clinics" which failed to distinguish between the many different types of stem cell. Those efforts have stalled, however. She stated that this was not a drug it was a simple procedure. Albini says the complications could have come from injecting a contaminant into the eye, or from the fact that the stem cells may have turned into myofibroblasts after the injections, which are cells associated with scarring.
The cases show that patients need to be warned that something that "sounds too good to be true may indeed be too good to be true and may even be disgusting", Albini says.
The company appeared to have plans to do more such procedures. Small molecule inhibitors for cancer stem cells in this study are available or being utilized in clinical trials for other diseases. The two women told Albini that they did not recall signing documents other than the single page form.
"Patients and physicians in the United States should be made aware that not all "stem cell" clinics are safe, and that "stem therapy" as provided in private clinics in the U.S. is unproven and potentially harmful", says Thomas Albini at the University of Miami's Bascom Palmer Eye Institute, Florida, who subsequently treated two of the women.
Each woman paid $5,000 for the procedure.
The doctors say patients should also check if anything purporting to be a clinical trial is affiliated with an academic medical centre and inform themselves about stem cell treatments.
"These women had fairly functional vision prior to the procedure. and were blinded by the next day", said ophthalmologist Dr Thomas Albini of the University of Miami, whose team examined the women after their treatment at a clinic in Florida. In the case of the eye injections, the clinic injected both eyes at once, which is highly unusual and unsafe for an experimental treatment.
The trial was not preceded by laboratory experiments and lacked comparison between treated patients and "control group" participants given a dummy therapy.
It's still not clear what went wrong, but the report says the treatment raised several red flags from the beginning.
But even if executed correctly, there is no evidence suggesting that the procedure could help restore vision, Goldberg and Albini said. A year later, the patient suffered none of the additional vision loss that would be common with the condition. iPS stem cells have a body of research behind their potential healing abilities, while the fat-based cells used in the procedures do not. It offers stem cell treatments for a variety of diseases and injuries, according to the company's website.
"With this new and exciting study, Dr. Wang and his team have provided the building blocks for understanding the cellular and genetic mechanisms behind squamous cell carcinoma", said Dr. Paul Krebsbach, dean of the UCLA School of Dentistry. "Doing the procedure in rats might have shown whether there are safety problems".
Without regulation of stem cell isolation and delivery processes at stem cell clinics, it's hard to know whether their cocktails contain harmful chemicals leftover from the isolation process - or whether they contain stem cells at all. In a regulatory filing, it reported that its techniques can treat chronic obstructive pulmonary disease, hip and knee conditions, diabetes, multiple sclerosis, Parkinson's disease, autism, colitis, and lupus.
Excerpt from:
Unproven Stem Cell Treatment Blinds 3 Florida Women - ClickLancashire
Man with 45% burns healed with stem cell treatment – ETHealthworld.com
By raymumme
Mumbai: A 45-year-old man -- suffering from 45 per cent burns due to a chemical spill at work -- has been healed with stem cell treatment, said the authorities at a hospital here on Friday.
Ram Naik (name changed) was brought to city-based StemRx Bioscience hospital after receiving first aid in another hospital. Nearly 45 per cent of his upper body was burned due to a chemical spill during work.
The impact of the burns led to a charred look on his face and body. Also, joint mobility due to the burns was reduced. The outer layer of the skin was affected, facial burns were of grade II level and in some instances grade III burns were also present, leading to deeper structures like the subcutaneous tissue also being affected.
According to the doctors, burn wound healing involves a series of complex processes, with healing time and scar tissue being the most important parameters that affect treatment outcomes. Burn injuries, especially severe ones, are proving to have devastating effects on the affected patients.
They said that stem cells have been recently applied in burn wounds to promote superior healing of the wounds. Not only have stem cells been shown to promote better and faster healing of the burn wounds, they are also capable of decreasing inflammation and prevent scar progression and fibrosis.
Therefore, the doctors decided to provide Naik stem cell treatment.
Regenerative Medicine researcher at Stemrx Bioscience hospital Pradeep Mahajan said that within two days, a notable improvement in his condition was observed and the swelling and charred appearance started reducing.
"Mild eyelid movements were noticed and on the third day the burns started drying on the face and he could open his mouth and eyes. Growth factors derived from platelets, cells, fibroblasts, collagen-based gel etc. was used during treatment. In addition, in areas with deep burns, sheets of PGLA coated with cells and growth factors were used," said Mahajan, adding that different medication and treatments were imparted and closed dressing was avoided.
"Blood transfusion and supplementary fluids were given intravenously to maintain systemic homeostasis," said Mahajan.
Stating that on 5th and 6th day following treatment, dry scales from the face and body started peeling off, the doctor's team also observed impressive changes such as new skin forming within a week of treatment with cells and growth factors.
By conventional modalities, it takes more than eight weeks for the patient to heal and many additional months for the patient to be able to regain joint and facial movements.
"By the 10th day of the treatment, dry scales completely peeled off and by the 14th day the patient had no tenderness or burning pain. Joint movements became free as well, Steady rate of progression of healthy skin formation is being noticed. Areas with deep burns are also healing at a rapid rate and I am confident that within a month we will accomplish thorough healing and the patient will be back to normal," Mahajan said.
Medical sciences say that such cases are challenging to manage considering the degree of impairment they result in due to prolonged healing period. Also, through conventional therapeutic modalities healing occurs with scar formation and results in contractures. Chances of systemic complications and infection are also high.
However according to the medical team, by using stem cells, the natural healing potential of the body is used, leading to reduction of healing time and promoting regeneration of affected tissues. This also reduces the mental trauma and financial burden that a patient goes through when under conventional management.
"Stem cell-based therapy has offered a novel and powerful strategy in almost every medical specialty including burns and wound management. Stem cells have proven to have tremendous potential in enhancing wound healing and facilitating skin regeneration," Mahajan said.
Go here to read the rest:
Man with 45% burns healed with stem cell treatment - ETHealthworld.com
Scientists Have Discovered A Secret Function Of Lungs – IFLScience
By raymumme
A good starting point for any scientist in any field is to acknowledge that theres a lot that we dont know. We dont know, for example, why there is more matter than antimatter in the universe. We dont know quite how the evolution of the dinosaurs panned out. And, perhaps most surprisingly of all, we dont know quite how many organs the human body has or what all their functions are.
This January, researchers have announced that a brand new organ had been discovered in our bodies after it had long been mistaken for something else. Now, writing in the journal Nature, a group of researchers from the University of California, San Francisco (UCSF), have found that the lungs in mice have a hidden feature too they help make blood.
Specifically, it appears the lungs produce over half of the platelets the components that bind blood together to stop us bleeding out when were wounded involved in circulation.
So not only do our breathing bags allow us to respire, but they also help keep our cardiovascular system full to the brim. Well thats rather lovely of them.
Thats not all. The researchers also managed to identify a cache of stem cells the type that can differentiate into almost any cell type with the right biological programming that can transform themselves into blood cells.
Bone marrow is thought to be the primary source of such stem cells, so this new revelation suggests that if our bone marrow is damaged and unable to keep up with its regular blood cell manufacture, our lungs can step in to make up for the shortfall.
This finding definitely suggests a more sophisticated view of the lungs that they're not just for respiration but also a key partner in formation of crucial aspects of the blood, senior author Mark Looney, a professor of medicine at UCSF, said in a statement.
A little caveat worth mentioning at this point is that this hasnt been directly imaged in humans, but mice. Nevertheless, the biological workings of these little critters is surprisingly similar to that of humans, which is part of the reason why theyre used in so many medical-themed studies so theres a good chance human lungs also possess the same hidden features.
Using a remarkable technique allowing the platelets to fluoresce, the team were able to directly trace the paths of the mousey platelets, and found they were coming from within the lungs. The megakaryocytes the platelet-producing cells are also seen moving back and forth between the lungs and the bone marrow, depending on where they are needed the most.
Perhaps studying abroad in different organs is a normal part of stem cell education, Looney added.
Visit link:
Scientists Have Discovered A Secret Function Of Lungs - IFLScience
Old blood can be made young again and it might fight ageing | New … – New Scientist
By raymumme
Fresh young cells
Dennis Kunkle Microscopy/Science Photo Library
By Jessica Hamzelou
BLOOD from the young seems to have healing powers, but how can we harness them without relying on donors? The discovery of a protein that keeps blood stem cells youthful might help.
The rejuvenating properties of young blood came to light in macabre experiments that stitched young and old mice together to share a circulatory system. The health of the older mice improved, while that of the younger ones deteriorated. Other animal studies have since shown that injections of young or old blood have similar effects.
This may work in people too. Young blood is being trialled as a treatment for conditions like Alzheimers, and aged mice that received injections of blood from human teenagers showed improved cognition, memory and physical activity levels.
We think the drug will improve signs of ageing and boost the immune systems of older people
But these studies rely on young people donating their blood: if this became the go-to therapy for age-related disease it would be difficult to get enough donations to fulfil demand.
The stem cells in our blood could provide an alternative approach. Our red and white blood cells are made by stem cells that themselves come from mother stem cells in bone marrow. But as we age, the number of these mother stem cells declines. One of the worlds longest-lived women seemed to only have two left in her blood when she died at age 115.
The decline in mother stem cells causes people to have fewer red blood cells, and white blood cells called B and T lymphocytes. These declines can cause anaemia and weaken the immune system. Usually the immune system in the elderly is not prepared to fight infections very hard, says Hartmut Geiger at the University of Ulm in Germany.
When Geigers team examined the bone marrow in mice, they found that older animals have much lower levels of a protein called osteopontin. To see if this protein has an effect on blood stem cells, the team injected stem cells into mice that lacked osteopontin and found that the cells rapidly aged.
But when older stem cells were mixed in a dish with osteopontin and a protein that activates it, they began to produce white blood cells just as young stem cells do. This suggests osteopontin makes stem cells behave more youthfully (EMBO Journal, doi.org/b4jp). If we can translate this into a treatment, we can make old blood young again, Geiger says.
Its exciting, says Hanadie Yousef at Stanford University in California. But longer term studies are needed to see whether this approach can rejuvenate the whole blood system, she says.
Until now, most efforts to use blood as a rejuvenation agent have focused on plasma, the liquid component, as some believe it carries dissolved factors that help maintain youth. But Geiger thinks the cells in blood might play a key role, because they are better able to move into the bodys tissues.
Both soluble factors and blood cells are likely to be important, says Yousef. While injections of young plasma rejuvenate older animals, the treatment doesnt have as strong an effect as when young and old animals share a circulatory system, she says.
Geigers team is developing a drug containing osteopontin and the activating protein to encourage blood stem cells to behave more youthfully. It should boost the immune system of elderly people, he says.
Such a drug might have benefits beyond fighting infection and alleviating anaemia. The team also think the protein will boost levels of mother stem cells. Having only a small number of such cells has been linked to heart disease, so Geiger says there is a chance that boosting them may help prevent this.
Osteopontin might also be useful for treating age-linked blood disorders, such as myelodysplasias that involve dysfunctional cells, says Martin Pera of the Jackson Laboratory in Bar Harbor, Maine. It is possible that rejuvenating bone marrow stem cells could help with these conditions, he says.
This study provides more evidence that cells can be rejuvenated, says Ioakim Spyridopoulos at Newcastle University, UK. They have made old blood look young again, although whether it acts young or not will have to be shown in clinical trials.
This article appeared in print under the headline Old blood made young again
More on these topics:
Go here to see the original:
Old blood can be made young again and it might fight ageing | New ... - New Scientist
76 Javakhk Armenians Join ABMDR as Bone Marrow Donors – Asbarez Armenian News
By raymumme
LOS ANGELESIn the course of March 3-4, the Armenian Bone Marrow Donor Registry (ABMDR) held unprecedented community-outreach and donor-recruitment events throughout Javakhk, in Western Georgia, led by an ABMDR team from Yerevan.
The historic recruitment campaign, which took place in Armenian communities in Akhaltskha, Akhalkalak, and Ninotsminda, was organized with the assistance of the Armenian Relief Society (ARS) of Javakhk, and the invaluable logistical support of Karine Tadevosyan, chairperson of the ARS Javakhk Region, and other local ARS members.
Throughout the recruitment and outreach events, ABMDR Executive Director Dr. Sevak Avagyan and Medical Director Mihran Nazaretyan delivered lectures and made presentations with regard to ABMDRs life-saving mission, to the great enthusiasm of hundreds of local Armenian-community members. Also addressing the community gatherings were Tadevosyan and other executive members of ARS Javakhk. By the conclusion of the recruitment campaign, 76 local Armenians had joined the ranks of ABMDR as potential bone marrow donors.
Words cannot describe our joy as we marvel at the support, excitement, and spirit of activism which our recruitment campaign was met with, in every single Javakhk community where we held events, said Dr. Frieda Jordan, President of ABMDR, and added, We convey our heartfelt gratitude to Karine Tadevosyan, all of her gracious ARS colleagues, other local community leaders, and the Armenian people of Javakhk as a whole, for joining our global family of bone marrow donors, toward our shared quest of saving lives.
About the Armenian Bone Marrow Donor Registry
Established in 1999, ABMDR, a nonprofit organization, helps Armenians and non-Armenians worldwide survive life-threatening blood-related illnesses by recruiting and matching donors to those requiring bone marrow stem cell transplants. To date, the registry has recruited over 28,000 donors in 42 countries across four continents, identified over 4,200 patients, and facilitated 27 bone marrow transplants. For more information, call (323) 663-3609 or visit abmdr.am.
View post:
76 Javakhk Armenians Join ABMDR as Bone Marrow Donors - Asbarez Armenian News
A Groundbreaking Stem Cell Treatment Just Prevented a Woman From Going Blind – Futurism
By raymumme
In Brief
Macular degeneration affects more than 10 million people in the U.S., and is the most common cause of vision loss. It is caused by the deterioration of the middle of the retina, called the macula. The macula focuses central vision and controls our ability to see objects in fine detail, read, recognize colors and faces, and drive a car. Until now, the disease has been considered incurable.
An octogenarian with the condition is now the first person to receivesuccessful treatmentwith induced pluripotent stem (iPS) cells. The progression of the womans macular degenerationwas arrested by new retinal cells made in the lab.Unlike embryonic stem cells, iPS cells can be created from regular adult cells.In this case, the cells used to repair the damaged retina from macular degeneration came from the womansskin.
The team at Kobe, Japans RIKEN Laboratory for Retinal Regeneration, led by Masayo Takahashi, created iPS cells from the patients skin cells. Then, theyencouraged them to form cells to patch the retinal pigment epithelium. These cells help nourish and support the retina, allowing it to capture the light the eye needs to see.
Once the cells were transformed, the team used them to make a slither measuring 1 by 3 millimeters. This was the patch they used to replace the diseased tissue removed from the patients retina. Their aim was to stop the degeneration and save her sight. The results show that the procedure was technically a success: although her vision did not improve, the degeneration stopped.
A possible concern about this treatment, however, is that creating new tissues from stem cells could cause genetic mutations, which might in turnlead to cancer. While more research in this area and its possible applications is needed, in the case of the patient at RIKEN, therehave been no signs of cancer or any other complications.
Read more from the original source:
A Groundbreaking Stem Cell Treatment Just Prevented a Woman From Going Blind - Futurism
Three Women Blinded In Stem Cell Clinical Trial – Vocativ
By raymumme
Three women suffering from a degenerative eye condition were blindedlikely permanentlyin a clinical trial for stem cell therapy, according to a report published Wednesday in the New England Journal of Medicine.
The women, who were all between the ages of 72 and 88, had a common medical condition called age-related macular degeneration, in which cells in the retina begin to die off, resulting in spotty or blurred vision. Researchers suspected stem cells derived from the patients own body could regenerate some of the cells lost to the disease. So in the clinical trial, which was conducted in 2015, researchers removed some blood and fat from participants anesthetized abdomens, treated the cells in a standardized way to make them revert to stem cells, then injected into their eyes. They were instructed to use an eyedrops antibiotic for a few days. The three patients had found the trial listed on the government web site clinicaltrials.gov, and had each paid $5,000 for the procedure. The informed consent form listed that blindness was possible as a result of the procedure.
A few days after the patients received the injected stem cells, the participants ended up in the hospital with vision loss, detached retinas, and hemorrhage. The patients lost vision; subsequent checkups led doctors to conclude that they would likely never regain their sight.
Despite the fact that the participants found the procedure on clinicaltrials.gov, the informed consent forms do not mention that it is in fact a clinical trial. The patients paid for a procedure that had never been studied in a clinical trial, lacked sufficient safety data, and was performed in both eyes on the same day, the study authors write. Injecting something experimental into both eyes is both not safe and not typical, they continue.
Recently researchers have been testing lots of different medical uses for stem cells, from treating multiple sclerosis to spinal cord injuries. With the passage of the 21st Century Cures Act in December, Congress cleared the way for faster regulatory approval for promising treatments based on stem cells. At least 13 clinical trials were registered to treat AMD alone as of November 2016, the article authors write.
But anecdotes like these bolster those who counsel restraint when it comes to stem cells. Although numerous stem-cell therapies for medical disorders are being investigated at research institutions with appropriate regulatory oversight, many stem-cell clinics are treating patients with little oversight and with no proof of efficacy, the article authors write.
Jeffrey Goldberg, a professor of ophthalmology at Stanford University and one of the authors of the article, calls this a call to awareness for patients, physicians and regulatory agencies of the risks of this kind of minimally regulated, patient-funded research, according to a press release.
See original here:
Three Women Blinded In Stem Cell Clinical Trial - Vocativ
Human Embryonic Stem (ES) Cells from Skin Cells …
By raymumme
The first new finding is an obvious onethe mouse experiments worked in human cells. Just because something worked in mice doesn't necessarily mean it will work in people too. So this is a really important finding.
The second important finding has to do with the specific genes each group used. Both groups added four genes to turn a stem cell into an ES cell. But they used a slightly different set of genes.
The Japanese group added OCT3/4, SOX2, KLF4, and c-MYC. The Wisconsin group added OCT4, SOX2, NANOG, and LIN28. This matters because of a side effect seen in the previous mouse study.
The mouse study went farther than the human study in that the researchers added these new ES cells to a mouse embryo. The results were disconcerting. Around 20% of the mice developed cancer from the cells. The researchers hypothesized that the cause was one or more of the genes that were used to create the ES cell.
By using different sets of genes in the human cell study, the researchers showed you don't need the same four genes to create an ES cell. The hope is that the researchers will find a combination of genes that do not cause cancer.
Once the scientists find a set of genes that don't cause cancer, this research should blow the stem cell field wide open. We still don't know if ES cells will work to actually cure disease. But ethical ES cells should open the spigot of federal funds so American scientists can finally research this subject to its full extent. Then we'll see if ES cells can really live up to their hype. Or if we need to pursue other ways to cure these illnesses.
Read the original here:
Human Embryonic Stem (ES) Cells from Skin Cells ...
Woodrose Ventures Corporation Announces Proposed Acquisition … – Marketwired (press release)
By raymumme
VANCOUVER, BRITISH COLUMBIA--(Marketwired - March 13, 2017) -
NOT FOR DISSEMINATION IN THE UNITED STATES
Editors Note: There is a photo associated with this press release.
Woodrose Ventures Corporation (TSX VENTURE:WRS.H) ("Woodrose" or the "Company") is pleased to announce that it has entered into an agreement (the "Agreement") dated March 10, 2017 to acquire all of the shares of Novoheart Holdings Ltd. ("Novoheart"), a global stem cell biotechnology company dedicated to human heart engineering (the "Transaction"). Novoheart develops products and provides services focused on engineering prototypes of bio-artificial human heart tissues and chambers for drug discovery, cardiotoxicity screening, disease modelling and therapeutic applications.
The Transaction will constitute a "reverse-takeover" of Woodrose in accordance with the policies of the TSX Venture Exchange (the "TSXV") and the reactivation of Woodrose, which is currently a NEX-listed issuer.
About Novoheart
Novoheart is a global stem cell biotechnology company headquartered in Hong Kong with R&D Innovation Centres being set up in the United States. Novoheart's mission is to revolutionize drug discovery and the development of heart therapeutics with its range of proprietary bioengineered human heart constructs, collectively known as the MyHeart platform, and to further develop them into transplantable heart grafts for cell-based regenerative therapies with superior safety and efficacy. Its scientific team has pioneered a range of best-in-class bioengineering technologies and constructed the world's first human mini-heart "novoHeart" with which the Novoheart team intends to revolutionize:
1) Pre-clinical drug discovery, cardiotoxicity screening and heart disease modelling;
2) Post-discovery, clinical development of novel therapeutics; and
3) Pre-clinical and clinical development of cell-based cardiac regenerative therapies.
Novoheart's immediate focus is to innovate and accelerate the lengthy, expensive and inefficient drug development process. The development of a new drug candidate typically costs US$2-4bn and takes 10+ years (Tufts Centre for the Study of Drug Development, Tufts CSDD R&D Cost Study 2014) with extremely poor success rates of
Novoheart's intellectual property portfolio, including the human "heart-in-a-jar" (novoHeart) and other related next-generation technologies of the MyHeart platform (see figure below) are unique solutions that help bridge the gap between pre-clinical and clinical drug trials. The MyHeart platform provides advanced human heart surrogates for pre-screening of drug formulas and the elimination of toxic compounds early on in the drug development process, minimizing the risk towards patients. Significantly, the MyHeart Platform provides real time data on the effects of drug formulations enabling drug development companies to undertake "on-the-fly" reformulation of drug candidates to optimize efficacy and toxicological profiles. With Novoheart's technologies, we aim to significantly reduce pre-clinical R&D time and costs, and importantly, improve trial successes. It is anticipated that drug screening results using Novoheart's human engineered tissues would be accepted as reliable indicators for toxicity and efficacy, thereby qualifying the test compounds for accelerated drug development.
Novoheart adopts a hybrid business model by:
These products and services are designed to significantly reduce the time, cost, and use of animal models, as well as improve patient safety, and facilitate pharmaceutical discovery and development. Novoheart is currently working with leading academic and pharmaceutical partners to innovate drug discovery and toxicity screening protocols. Our targeted clients are pharmaceutical companies, government units, and research institutions.
Novoheart was incorporated in 2014 pursuant to the laws of British Virgin Islands (BVI) and its controlling shareholder is Medera Group Limited, a BVI entity. Novoheart has one wholly owned Hong Kong subsidiary "Novoheart Limited" ("Novoheart Hong Kong") which is the group operating entity.
Novoheart Hong Kong was incorporated in January 2014 by founder and CEO Prof. Ronald Li, with scientific co-founders Prof. Kevin Costa and Prof. Michelle Khine.
Novoheart's foundational technologies are the direct outcome of over 15 years of research effort supported by R&D investments amounting to approximately USD30MM. These research efforts, performed at Johns Hopkins University, Icahn School of Medicine at Mount Sinai, University of California Irvine, University of California Davis, and the University of Hong Kong by our scientific founders, have received major recognitions such as American Heart Association's Best Study of 2005, Ground-breaking Study of 2006, and Late-breaking Studies of 2002, 2003, 2005 and 2007, and the Spirit of Hong Kong Innovating for Good Award in 2015. The "human-heart-in-a-jar" technology was selected by Google's Solve For X as a Moonshot Project in 2015.
Novoheart's scientific founders and advisors are renowned pioneering leaders in the stem cell and cardiac space, with a successful track record in developing and commercializing ground-breaking technologies. In September 2014, Novoheart established its R&D base and office in the Hong Kong Science Park, where it continues to innovate solutions for drug discovery and human heart tissue engineering.
In December 2014, Novoheart signed a strategic partnership with a major global pharmaceutical company (the "Global Pharma Partner") headquartered in New York City to validate the MyHeart platform. The success of this validation process has resulted in follow on income-generating projects.
In January 2015, Novoheart's R&D proposal to develop bio-artificial heart tissues for drug screening received 50/50 matched funding from the Innovation & Technology Commission (ITC) of the Government of Hong Kong, with a total project cost of over HK$21MM over 2 years. It was also the largest biotech project granted by ITC for that year. Novoheart owns all of the intellectual property generated from this project, and as a result of the R&D, Novoheart has applied or is in the application process for 3 new patents covering newly developed technology, including the human ventricular cardiac anisotropic sheet (hvCAS) as a powerful tool for detecting drug-induced arrhythmias with the results published in the prestigious international peer-reviewed bioengineering journal Advanced Materials (Shum et al. 2017, Advanced Materials, 29). Additionally, Novoheart holds exclusive worldwide licenses or options to acquire the same for technologies that constitute its MyHeart platform and future developments.
In December 2015, Novoheart signed a second contract with the Global Pharma Partner to build disease-specific engineered human heart tissues and chambers for drug discovery. The total project cost is US$726,000 over 1.5 years.
In February 2017, the Corporate Venture Fund (CVF) of the Hong Kong Science and Technology Parks Corporation (HKSTPC) completed an equity investment of approximately US$250,000 into Novoheart and an additional investment would be made at the Transaction.
Novoheart Financial Information
The following table includes a summary of certain financial information of Novoheart and is derived from its financial statements for the years ended June 30, 2016 and June 30, 2015.
Summary of the Transaction
Under the terms of the Agreement, the shareholders of Novoheart will receive an aggregate of 66,086,600 common shares of Woodrose on a post-Consolidation basis (see below) ("Woodrose Post-Consolidation Shares"). In addition, a finder's fee of 2,313,038 Woodrose Post-Consolidation Shares will be paid to Cynosure Private Equity Limited in connection with the Transaction.
In connection with the Transaction, Woodrose intends to complete a consolidation of all its outstanding common shares on the basis of 3.56878449 old common shares for each one new common share (the "Consolidation"). In addition, Woodrose intends to complete a non-brokered private placement (the "Private Placement") of 11,700,000 subscription receipts ("Subscription Receipts") at a price of CDN$0.50 per Subscription Receipt to raise gross proceeds of CDN$5,850,000, which will be held in escrow in accordance with the terms of a subscription receipt agreement (the "Subscription Receipt Agreement"). It is anticipated that the Subscription Receipt Agreement will provide that, upon completion of the Transaction, each Subscription Receipt will automatically convert into one Woodrose Post-Consolidation Share. The Subscription Receipt Agreement will also provide that, in the event the Transaction is terminated or does not complete within an agreed timeframe, the Subscription Receipts will be cancelled and the funds will be returned to the holders. Woodrose may pay cash fees in an amount not to exceed 7% of the gross proceeds (to a maximum of $364,000) to certain finders involved in the Private Placement and may issue finder's warrants ("Finder's Warrants"), in an amount not to exceed 7% of the number of Subscription Receipts issued (to a maximum of 728,000 Finders Warrants) each of which would entitle the holder to acquire one Woodrose Post-Consolidation Share at a price of CDN$0.50 for a period of two years following closing of the Private Placement. All securities issued pursuant to the Private Placement will be subject to a statutory hold period of four months and one day.
The Company intends to use the net proceeds of the offering to finance investment in drug discovery and screening, establish commercial partnerships, expand the current laboratory, hire additional research and development team members and for working capital and general corporate purposes.
Upon completion of the Transaction, it is anticipated that the Company will be classified as a Tier 2 Technology Issuer on the TSXV and will change its name to "Novoheart Holdings (BC) Limited" or such other name as is acceptable to the Board of Directors. Closing of the Transaction ("Closing") is subject to conditions precedent, that include, but are not limited to, the following:
The Transaction is an "arm's length" transaction (as defined by the policies of the TSXV). Woodrose intends to rely an exemption from the sponsorship requirements of the policies of the TSXV.
Proposed Management Team
Upon closing of the Transaction, the following directors and senior officers are anticipated to be appointed in replacement of Woodrose's current board and management:
Prof. Ronald Li, B.Sc. (Hons), Ph.D. (Proposed President, Chief Executive Officer and Director)
Prof. Ronald Li is a co-founder of Novoheart, and has been serving as the CEO since 2016. He is concurrently Director of Ming-Wai Lau Centre for Reparative Medicine, HK node, Karolinska Institutet (KI), Sweden, with a professorial cross appointment at the Dr. Li Dak-Sum Research Centre, The University of Hong Kong (HKU)-KI Collaboration in Regenerative Medicine of HKU. Prof. Li has been an advocate of stem cell technology for many years, starting from his career as Assistant Professor of Cardiology, and Cellular and Molecular Medicine at the Johns Hopkins University (JHU) School of Medicine. He founded and led the Human Embryonic Stem Cell Consortium when he was recruited in 2005 to become a tenured Associate Professor at the University of California, Davis, in light of state's USD3-billion stem cell initiative Proposition 71. Prof. Li was the Founding Director of the Stem Cell & Regenerative Medicine Consortium (SCRMC) at the University of Hong Kong (HKU) from 2010 to 2015. He also co-directed the Section of Cardiovascular Cell & Tissue Engineering in Icahn School of Medicine at Mount Sinai with Prof. Kevin Costa. Prof. Li has received multiple accolades and recognitions during his career, including the Spirit of Hong Kong Innovating for Good Award by the South China Morning Post (2015), the Top Young Faculty Award (2002, 2004), the Top Prize for the Young Investigator Basic Research (2001) and Top Postdoctoral Fellow Helen Taussig Award (2001) of JHU School of Medicine, Young Investigator Award 1st Prize from the Heart Rhythm Society (2002), and the Career Development Award from the Cardiac Arrhythmias Research & Education Foundation (2001).
Prof. Li graduated with his B.S. with honors in Biotechnology from University of Waterloo, Ontario, on Dean's List and his Ph.D. in Cardiology/Physiology at the University of Toronto.
Dr. Camie Chan, B.Sc. (Hons), M.Sc., Ph.D. (Proposed Chief Operating Officer and Director)
Dr. Camie Chan joined Novoheart Hong Kong as the Chief Operating Officer in 2016, after having served at HKU as the Deputy Director of the Faculty of Medicine Core Facility, a founding member of the Management Committee of the Stem Cell & Regenerative Medicine Consortium (SCRMC), and Assistant Professor in the Department of Anatomy, between 2010 and 2016. She has had extensive experience managing laboratory operations in her capacity at HKU, and her prior career as Assistant Professor at the University of California, Davis, and Assistant Investigator at the Shriners Hospital for Children. Dr. Chan is also a co-inventor of technology allowing mass production of human ventricular heart cells from pluripotent stem cells.
Dr. Chan graduated with her B.Sc. with honors at the University of Waterloo, followed by obtaining her M.Sc. degree in Medical Sciences and Ph.D. degree in Immunology at the University of Toronto, Canada. She then received postdoctoral training at the Sydney Kimmel Cancer Research Center at the Johns Hopkins University. She has garnered numerous awards in her career, including the prestigious National Institute of Allergy and Infectious Diseases (NIAID) Developmental Research Grant Award.
Prof. Kevin Costa, B.S., Ph.D. (Proposed Chief Scientific Officer)
Prof. Costa is Director of the Section of Cardiovascular Cell and Tissue Engineering at the Icahn School of Medicine at Mount Sinai in New York City. Prof. Costa was previously trained at the Johns Hopkins University and on the faculty as Associate Professor of Biomedical Engineering at Columbia University. As a "blue-blood" biomedical engineering (BME) expert (B.S. and M.S. in BME from Boston University, Ph.D. in BME from UC San Diego, and postdoc in BME from JHU and University of Washington) in cell and tissue biomechanics and cardiac tissue engineering, he has developed one of the first engineered cardiac tissue systems. Since 2009, he has been working with Prof. Ronald Li to translate such systems into human cells. Prof. Costa has received research funding from the Whitaker Foundation, the National Science Foundation (NSF) and the National Institutes of Health (NIH; NHLBI, NIBIB, and NIGMS). He was also a recipient of the prestigious Faculty Early Career Development (CAREER) Award from the NSF. Prof. Costa is an inventor of several cardiac tissue engineering technologies and one of the scientific co-founders of Novoheart Hong Kong.
Ms. Iris Lo, B. Comm. (Hons), CPA, CA (Proposed Chief Financial Officer)
Ms. Lo is a seasoned professional with expertise in corporate finance, mergers and acquisitions, accounting, and finance. Prior to joining Novoheart, Ms. Lo was the Director of Corporate Development & Analysis at Cardiome Pharma Corp., a Canadian public company dually listed on the TSX and NASDAQ (TSX: COM, NASDAQ: CRME). At Cardiome, she held responsibilities in equity and debt financing, corporate mergers and acquisitions, product licensing and distributions, financial planning and analysis, as well as regulatory and risk management. During her tenure at Cardiome, Ms. Lo participated in transactions totaling over US$240 million as Cardiome grew from a company with a market capitalization of US$25 million to over US$150 million at its peak. She brings with her valuable experience from the life sciences and pharmaceutical sector, as well as expertise in dealing with the complexities of operating and financing public corporations. Ms. Lo was also previously a Manager in the Transaction Services team at PwC Hong Kong and began her career articling with KPMG Vancouver. She is a Chartered Professional Accountant and holds a Bachelor of Commerce (Honours) from the Sauder School of Business at the University of British Columbia.
Mr. Victor Chang (Proposed Director)
Mr. Chang is a seasoned investor who has lately become focused on start-ups. Mr. Chang started his career with Lippo Securities Limited in 1996 and became a Director of Grand International Holdings Limited in 1999, which was engaged in general investments. During the period from 2007 to 2009, he was a Director and Responsible Officer for Astrum Capital Management Limited carrying out regulated activities under the Securities and Futures Ordinance ("SFO", Cap. 571, Laws of Hong Kong) and with Murtsa Capital Partners Limited as well. During the period from 2007 to 2012, he was also a compliance consultant for Astrum Capital Management Limited. As co-founder and Managing Director of Zebra Strategic Outsource Solution, he has over 16 years of experience in recruitment process outsourcing, executive search as well as and private investment management. In Apil 2013, he successfully brought Zebra Strategic Holdings Limited which offers holistic HR solutions to IPO on the HK GEM board (Stock Code: 8260) and was re-designated as and is currently a Non-Executive Director with the company. He is currently a Director and Responsible Officer of Dakin Financial Group, a corporation licensed to carry out type 1, 2 & 9 regulated activity under the Hong Kong Securities and Futures Ordinance.
Mr. Tong Ricky Chiu (Proposed Director)
As a key founder and visionary for Grand Power Logistics Group Inc., which was listed on the TSX Venture Exchange (GPW.V) before its privatization in 2016, and Baoshinn International Express Ltd., Mr. Chiu adds value with his immense corporate development and growth skills. He received his education in Oxford University, England, and Beijing University, and began his career in Australia. He has a diversified background in a wide range of industries with roles in finance, audit, real estate, merchandise trading and travel, as well as logistics.
Mr. James Topham (Proposed Director)
Mr. Topham is an experienced executive with expertise in finance, accounting, auditing and entrepreneurial technology companies. He was an audit partner leading KPMG's Technology Group in the Vancouver office for 20 years where he worked with many fast growing public companies and was involved in many M&A and IPO transactions in Canada, the US and Europe. Mr. Topham founded Social Venture Partners Vancouver in 2001 with a mission to strengthen the organizational capacity of innovative non-profits serving children in-need and youth at-risk. It has funded several million dollars and provided thousands of hours of executive time mentoring these local non-profits. Since retiring at KPMG 7 years ago, Mr. Topham has worked on several Boards of both public and private technology companies. He received a lifetime achievement award from the BC Technology Industry Association and was awarded the designation of Fellow Chartered Public Accountant (FCPA) from the Chartered Public Accountants of BC for his career achievements in the profession and community. He was a founder and Board member for 9 years of the BC Technology Industry Association that represents the technology industry in BC. Mr. Topham is a CPA and has a Bachelor of Commerce degree with Honours from the University of Saskatchewan graduating as the most distinguished graduate in the College of Commerce.
Mr. Allen Ma (Proposed Director)
As a 30-year technology industry veteran, Mr. Ma was the CEO of Hong Kong Science & Technology Parks before he retired in July 2016. He held senior executive positions within the information and communications technology sector. His past roles include president for Asia-Pacific at British Telecom, vice-president for Asia at the global telecom solutions sector of Motorola, executive director of Hong Kong Telecommunications - subsequently called Cable & Wireless HKT - and managing director of Hong Kong Telecom CSL. Ma holds an MBA from the University of Toronto and is a fellow member of both the Chartered Institute of Management Accountants, UK and the Association of Chartered Certified Accountants, UK. He is also a Certified Management Accountant of Canada.
Proposed Advisory Team
Novoheart is supported by a Scientific Advisory Board whose proposed composition consists of eminent scientists renowned in the fields of stem cells, cardiac biology and physiology, tissue engineering, and clinical cardiology including clinical trials research, from top academic research institutes in the U.S.A. Their technical expertise will guide the development of Novoheart as a forerunner in the application of cutting-edge technologies to develop new and better treatments for heart disease and beyond.
Further Details
Both the Company and Novoheart intend to work diligently to complete the conditions precedent to Closing and anticipate completion of the Transaction in the second quarter of 2017. The Company will update its shareholders with further details as they become available.
ON BEHALF OF WOODROSE VENTURES CORPORATION
Darren Devine, President, CEO and Director
NEITHER THE TSX VENTURE EXCHANGE NOR ITS REGULATION SERVICES PROVIDER (AS THAT TERM IS DEFINED IN THE POLICIES OF THE TSX VENTURE EXCHANGE) ACCEPTS RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
Completion of the Transaction is subject to a number of conditions, including but not limited to, Exchange acceptance and if applicable pursuant to Exchange requirements, majority shareholder approval. Where applicable, the Transaction cannot close until the required shareholder approval is obtained. There can be no assurance that the Transaction will be completed as proposed or at all.
Investors are cautioned that, except as disclosed in the Filing Statement to be prepared in connection with the Transaction, any information with respect to the Transaction may not be accurate or complete and should not be relied on. Trading in securities of the Company should be considered highly speculative.
The TSX Venture Exchange has in no way passed upon the merits of the Transaction and has neither approved nor disproved the contents of this news release.
Cautionary Note Regarding Forward-Looking Statements
Information set forth in this news release may involve forward-looking statements under applicable securities laws. Forward-looking statements are statements that relate to future, not past, events. In this context, forward-looking statements often address expected future business and financial performance, and often contain words such as "anticipate", "believe", "plan", "estimate", "expect", and "intend", statements that an action or event "may", "might", "could", "should", or "will" be taken or occur, or other similar expressions. All statements, other than statements of historical fact, included herein including, without limitation; statements about the terms and completion of the Transaction are forward-looking statements. By their nature, forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements, or other future events, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Such factors include, among others, the following risks: failure to satisfy all conditions precedent to the Transaction, including shareholder approval, approval of the TSX Venture Exchange and completion of the necessary financings and the additional risks identified in the management discussion and analysis section of Woodrose Corporation's interim and most recent annual financial statement or other reports and filings with the TSX Venture Exchange and applicable Canadian securities regulators. Forward-looking statements are made based on management's beliefs, estimates and opinions on the date that statements are made and the respective companies undertakes no obligation to update forward-looking statements if these beliefs, estimates and opinions or other circumstances should change, except as required by applicable securities laws. Investors are cautioned against attributing undue certainty to forward-looking statements.
To view the photo associated with this press release, please visit the following link: http://www.marketwire.com/library/20170312-1088577_MyHeart_800.jpg
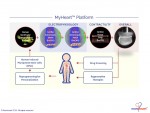
Read the original:
Woodrose Ventures Corporation Announces Proposed Acquisition ... - Marketwired (press release)
TiGenix Announces Positive Topline Week-104 Data for Cx601 ADMIRE-CD Trial – P&T Community
By raymumme
TiGenix Announces Positive Topline Week-104 Data for Cx601 ADMIRE-CD Trial P&T Community Effective July 31, 2015, TiGenix acquired Coretherapix, whose lead cellular product candidate, AlloCSC-01, is currently in a Phase II clinical trial in Acute Myocardial Infarction (AMI). In addition, the second product candidate from the cardiac stem ... |
See the original post here:
TiGenix Announces Positive Topline Week-104 Data for Cx601 ADMIRE-CD Trial - P&T Community
Artificial embryo grown in a dish from two types of stem cells – New Scientist
By raymumme
By Andy Coghlan
Sarah Harrison and Gaelle Recher, Zernicka-Goetz Lab, University of Cambridge
Artificial mouse embryos grown from stem cells in a dish could help unlock secrets of early development and infertility that have until now evaded us.
Magdalena Zernicka-Goetz at the University of Cambridge and her team made the embryos using embryonic stem cells, the type of cells found in embryos that can mature into any type of tissue in the body.
The trick was to grow these alongside trophoblast stem cells, which normally produce the placenta. By growing these two types of cell separately and then combining them in a special gel matrix, the two mixed and started to develop together.
After around four-and-a-half days, the embryos resembled normal mouse embryos that were about to start differentiating into different body tissues and organs.
They are very similar to natural mouse embryos, says Zernicka-Goetz. We put the two types of stem cells together which has never been done before to allow them to speak to each other. We saw that the cells could self-organise themselves without our help.
This is the first time something resembling an embryo has been made from stem cells, without using an egg in some way. Techniques such as cloning, as done for Dolly the sheep and other animals, bypass the need for sperm, but still require an egg cell.
The artificial embryos are providing new insights into how embryos organise themselves and grow, says Zernicka-Goetz. The team engineered the artificial embryos so the cell types fluoresced in different colours, to reveal their movements and behaviour as the embryos go through crucial changes.
Mammal embryos were already known to start as a symmetrical ball, then elongate, form a central cavity and start developing a type of cell layer called mesoderm, which ultimately goes on to form bone and muscle.
We didnt know before how embryos form this cavity, but weve now found the mechanism for it and the sequential steps by which it forms, says Zernicka-Goetz. Its building up the foundations for the whole body plan.
The work is a great addition to the stem cell field and could be extended to human stem cell populations, says Leonard Zon at Boston Childrens Hospital, Massachusetts. Using the system, the factors that participate in embryo development could be better studied and this could help us understand early events of embryogenesis.
But Robin Lovell-Badge at the Francis Crick Institute in London says that the embryos lack two other types of cell layer required to develop the bodies organs: ectoderm, which forms skin and the central nervous system, and endoderm, which makes our internal organs.
Zernicka-Goetz hopes to see these types of cell layers develop in future experiments by adding stem cells that normally form the yolk sac, a third structure involved in embryonic development, to the mix.
If a similar feat can be achieved using human stem cells, this could tell us much about the earliest stages of our development. Current research is limited by the number of excess embryos that are donated from IVF procedures. But the new technique could produce a limitless supply, making it easier to conduct in-depth research. These artificial embryos may also be easier to tinker with, to see what effect different factors have in early embryogenesis.
Disrupting development in this way may provide new insights into the causes of abnormal embryo development and miscarriage. You would be able to understand the principles that govern each stage of development. These are not normally accessible, because they happen inside the mother, says Zernicka-Goetz.
But it is doubtful that this work could ever lead to fully grown babies in the lab. Lovell-Badge says the artificial embryos are unlikely to develop in vitro much further than shown in the study, as they would soon need the supply of nutrients and oxygen that a placenta normally channels from the mother.
Were not planning to make a mouse in the lab using stem cells, says Zernicka-Goetz. But she is hopeful that adding yolk sac stem cells will allow these artificial embryos to survive long enough to study the beginnings of organs like the heart.
Journal reference: Science, DOI: 10.1126/science.aal1810
Read more: Its time to relax the rules on growing human embryos in the lab
More on these topics:
Read more:
Artificial embryo grown in a dish from two types of stem cells - New Scientist
Stem Cells and Aging | Life Code
By raymumme
Adult stem cell function declines with age leading to the decline in fitness
The potential therapeutic use of stem cells is a very hot topic these days. Most of the attention has focused on embryonic stem cells and induced Pluripotent Stem cells (iPS cells), which can form every tissue type in the body to regenerate failing organs. The problem is that detailed knowledge is lacking for how to stimulate the embryonic stem cells to form differentiated tissues (e.g. cells that form the heart, pancreas, muscle, and brain). Moreover, because embryonic stem cells are unlimited in their ability to form any type of tissue, the risk of cancer looms large over the therapeutic use of embryonic stem cells. For example, both embryonic and IPS stem cells can form tumors called teratomas when injected into immune-compromised mice. Enter the bodys adult stem cells, which have not generally been associated with cancer and have been used safely as therapeutics in many countries. The problem with adult stem cells is that it is difficult to get enough of them to be effective for most indications or target the harvested adult stem cells to the proper tissue. Moreover, there are scores of different types of adult stem cells in the body, so picking the best type of adult stem cell for a particular therapeutic can be challenging. Thus, adult stem cell therapeutics with all its potential to regenerate damaged organs and tissues is still a work in progress.
But what about the many populations of endogenous adult stem cells that everyone has embedded in every organ system of the body? All the organs and differing tissues of the body appear to have adult stem cells available for regenerating cells in case of injury or disease. It was recently discovered that even brain neurons and heart muscle cells (previously thought to be non-dividing and irreplaceable in adults) have their own reservoirs of adult stem cells for regeneration. Unfortunately, as we age most adult stem cell populations either decline in number and/or lose the ability to differentiate into functional tissue-specific cells. For example, cardiac muscle stem cells exist but old folks have only one half the number of cardiac stem cells found in young people. Thus, adult stem cells become more and more dysfunction with age, which progressively increases organ and tissue dysfunction with age.
There are many examples revealing the role of adult stem cells in aging. First, the outer surface of your skin continuously sloughs off dead cells, so that adult stem cells must continuously replenish the dying skin cells to maintain the skin as an effective protective barrier to the outside world. With age, there are progressively fewer functional skin stem cells, so cell turnover in the skin slows, leading to thinner, dryer skin that loses its elasticity and youthful beauty. Second, hair also thins and goes grey, as functional follicle stem cell decline and the adult stem cells generating hair color also decline. Third, the differing adult stem cells that maintain the tissues composing skeletal muscle, pancreas, heart, bone, liver, kidney, and the immune system lose functional capacity, raising the potential for decline in tissue function or outright failure with age. As a final example, the five senses of sight, hearing, smell, taste, and touch slowly wane with age, as the declining stem cell populations responsible for maintaining these functions are unable to fully replenish the sensory neurons after injury and random cell death.
If your own adult stem cells are a key factor in aging and disease, then one novel way to slow aging and disease is to stimulate your own adult stem cells to maintain their proper numbers and functional capacity to differentiate into the various tissues as needed for repair and regeneration. This makes sense, because in most, if not all, organs of the body, old cells are continually being replaced by new cells coming from the adult stem cell populations. If stem cells are not producing enough new cells, then organs slowly decline in function as you age. Thus, stimulating your own stem cells can be a winning strategy to stave off many of the disorders associated with aging.
In practice, however, stimulating adult stem cell populations in the body is not a simple task. If the proliferation of adult stem cells is over stimulated, then one may get overgrowth of tissues or a potential tumor. Alternatively, one may stimulate the stem cells to proliferate in a balanced and regulated way, but the stem cells lose functionality and cannot differentiate into the desired specialized tissues to replace senescent cells. These twin problems promoting over stimulation or dysfunctional stem cells put real limits on any proposed therapeutic for stimulating stem cells. For example, most current treatments to stimulate immunity or stem cells (nave T cells) rely on complex carbohydrates from mushrooms or microorganisms to provide antigenic material that can stimulate immunity. This will activate the immune system stem cells to make more differentiated non-stem memory T cells directed against the antigenic material, but it does nothing to stimulate more immune stem cells (nave T cells). Indeed, chronic use of such stem cell enhancers may actually lead to stem cell depletion, as more adult stem cells are exhausted from the requirement to respond to the constant presence of the polysaccharide antigen. Indeed, one theory of how the HIV virus causes a defective immune system is that it exhausts the supply of nave T cells by the repeated attacks of the mutating HIV virus.
Stem Cell 100TM is a nutraceutical supplement that improves the function of your existing stem cells rather than over stimulate stem cells to differentiate or divide. By promoting the stability and vitality of adult stem cells they have the capacity to divide when the body signals a need for more stem cells and differentiated cells. When an organ or tissue is damaged, it will send out natural signals that new cells are needed to replace old or damaged cells. Stem Cell 100TM allows the adult stem cells to respond to the damage signal by provided new differentiated cells to replace the old damaged cells and also make more adult stem cells to keep up the stem cell population. Two other compounds in Stem Cell 100TM provide further natural support for stem cells.
(Note that not everyone will experience the same effects, as conditions vary among individuals. The general expectation is that for most health measurements that are in the Normal Range for your age, Stem Cell 100TM will promote readings that you had when some 20 years younger.)
The statements above have not been reviewed by the FDA. Stem Cell 100TM is not meant as a preventive or treatment for any disease.
See the original post here:
Stem Cells and Aging | Life Code


 April 8th, 2017
April 8th, 2017