Auxly Announces Upsized Bought-Deal Public Offering to $17.5 Million
By Dr. Matthew Watson
NOT FOR DISTRIBUTION TO U.S. NEWSWIRE SERVICES OR DISSEMINATION IN THE UNITED STATES
Originally posted here:
Auxly Announces Upsized Bought-Deal Public Offering to $17.5 Million
Trishula Therapeutics Appoints Anil Singhal as Chief Executive Officer
By Dr. Matthew Watson
To Read More: Trishula Therapeutics Appoints Anil Singhal as Chief Executive OfficerMDxHealth Launches Capital Increase and Provides Preliminary 2020 Financial Results
By Dr. Matthew Watson
THIS ANNOUNCEMENT IS NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, AUSTRALIA, CANADA, JAPAN, SOUTH AFRICA OR ANY OTHER JURISDICTION WHERE TO DO SO WOULD BE PROHIBITED BY APPLICABLE LAW
See the original post here:
MDxHealth Launches Capital Increase and Provides Preliminary 2020 Financial Results
DEINOVE strengthens its Business development team to deploy its partnering strategy
By Dr. Matthew Watson
DEINOVE strengthens its Business development team to deploy its partnering strategy
Read more:
DEINOVE strengthens its Business development team to deploy its partnering strategy
Hollister Biosciences Inc. Announces an Increase in Private Placement to $6.5 Million
By Dr. Matthew Watson
** THIS NEWS RELEASE IS INTENDED FOR DISTRIBUTION IN CANADA ONLY AND IS NOT INTENDED FOR DISTRIBUTION TO UNITED STATES NEWSWIRE SERVICES OR DISSEMINATION IN THE UNITED STATES.**
Read the original:
Hollister Biosciences Inc. Announces an Increase in Private Placement to $6.5 Million
Novavax Names Madelyn Caltabiano Senior Vice President, Global Program Management
By Dr. Matthew Watson
GAITHERSBURG, Md., Jan. 22, 2021 (GLOBE NEWSWIRE) -- Novavax, Inc. (Nasdaq: NVAX), a late-stage biotechnology company developing next-generation vaccines for serious infectious diseases, today announced the appointment of Madelyn ‘Lyn’ Caltabiano, Ph.D. to the position of Senior Vice President, Global Program Management. In this newly created role, Dr. Caltabiano will lead and expand the Global Program Management organization for the company’s pipeline and will develop appropriate strategies to assess the company’s operational performance, including areas related to portfolio valuation, milestone decision making and prioritization. She will report directly to Stanley C. Erck, President and Chief Executive Officer.
See original here:
Novavax Names Madelyn Caltabiano Senior Vice President, Global Program Management
Zealand Pharma announces update to 2020 financial guidance
By Dr. Matthew Watson
Company announcement – No. 1 / 2021
Read the original post:
Zealand Pharma announces update to 2020 financial guidance
Blood Sugar Premier – Does This Supplement Really Works? Ingredients & Blood Sugar Premier Reviews by Nuvectramedical
By Dr. Matthew Watson

Read more here:
Blood Sugar Premier – Does This Supplement Really Works? Ingredients & Blood Sugar Premier Reviews by Nuvectramedical
Bespoke Extracts Announces $100K Investment From CEO and Addition of New Brand Ambassador, UFC Fighter Maryna Moroz
By Dr. Matthew Watson

Read more here:
Bespoke Extracts Announces $100K Investment From CEO and Addition of New Brand Ambassador, UFC Fighter Maryna Moroz
Ulcerative Colitis Phase II Study of GSK2831781 Discontinued
By Dr. Matthew Watson
Immutep’s Collaboration and Exclusive License with GSK Remains in Place Immutep’s Collaboration and Exclusive License with GSK Remains in Place
See the original post here:
Ulcerative Colitis Phase II Study of GSK2831781 Discontinued
MDxHealth Successfully Completes a EUR 25.0 Million (USD 30.4 Million)(1) Capital Increase
By Dr. Matthew Watson
THIS ANNOUNCEMENT IS NOT FOR DISTRIBUTION, DIRECTLY OR INDIRECTLY, IN THE UNITED STATES OF AMERICA, AUSTRALIA, CANADA, JAPAN, SOUTH AFRICA OR ANY OTHER JURISDICTION WHERE TO DO SO WOULD BE PROHIBITED BY APPLICABLE LAW
Read more from the original source:
MDxHealth Successfully Completes a EUR 25.0 Million (USD 30.4 Million)(1) Capital Increase
Icosapent Ethyl Included in the Chinese Society of Cardiology (CSC) Updated Guidelines for Primary Prevention of Cardiovascular Diseases
By Dr. Matthew Watson
DUBLIN, Ireland and BRIDGEWATER, N.J., Jan. 21, 2021 (GLOBE NEWSWIRE) -- Amarin Corporation plc (NASDAQ:AMRN) today announced that the Chinese Society of Cardiology (CSC) has included icosapent ethyl in its updated Guidelines for Primary Prevention of Cardiovascular Diseases for 2021 as published in the Chinese Journal of Cardiovascular Diseases. The guideline authors include “icosapent ethyl 2 grams twice a day (as studied in REDUCE-IT®) as a treatment consideration to further lower atherosclerotic cardiovascular disease (ASCVD) in the appropriate patient population.”1
See the original post here:
Icosapent Ethyl Included in the Chinese Society of Cardiology (CSC) Updated Guidelines for Primary Prevention of Cardiovascular Diseases
Immutep Quarterly Activities Report
By Dr. Matthew Watson
To Read More: Immutep Quarterly Activities ReportKiadis announces multiple abstracts related to its K-NK-cell therapy platform have been accepted for presentation at TCT, the Combined Transplantation…
By Dr. Matthew Watson
Amsterdam, The Netherlands, January 22, 2021 – Kiadis Pharma N.V. ("Kiadis" or the "Company") (Euronext Amsterdam and Brussels: KDS), a clinical-stage biopharmaceutical company developing innovative NK-cell-based medicines for the treatment of life-threatening diseases, today announces that four abstracts related to its K-NK-cell therapy platform were accepted for presentation at the TCT Meetings, the Transplantation & Cellular Therapy Meetings of the American Society of Transplantation and Cellular Therapy (ASTCT) and Center for International Blood & Marrow Transplant Research (CIBMTR), which is being held virtually from February 8–12, 2021.
ONO Receives a Manufacturing and Marketing Approval of Adlumiz® (Anamorelin), a Ghrelin Receptor Agonist for the Treatment of Cancer Cachexia in…
By Dr. Matthew Watson
To Read More: ONO Receives a Manufacturing and Marketing Approval of Adlumiz® (Anamorelin), a Ghrelin Receptor Agonist for the Treatment of Cancer Cachexia in…Nicox Analyst Coverage Initiated by Edison Investment Research
By Dr. Matthew Watson
Go here to see the original:
Nicox Analyst Coverage Initiated by Edison Investment Research
BioCryst Announces Approval of ORLADEYO™ (berotralstat) in Japan for the Prophylactic Treatment of Hereditary Angioedema
By Dr. Matthew Watson
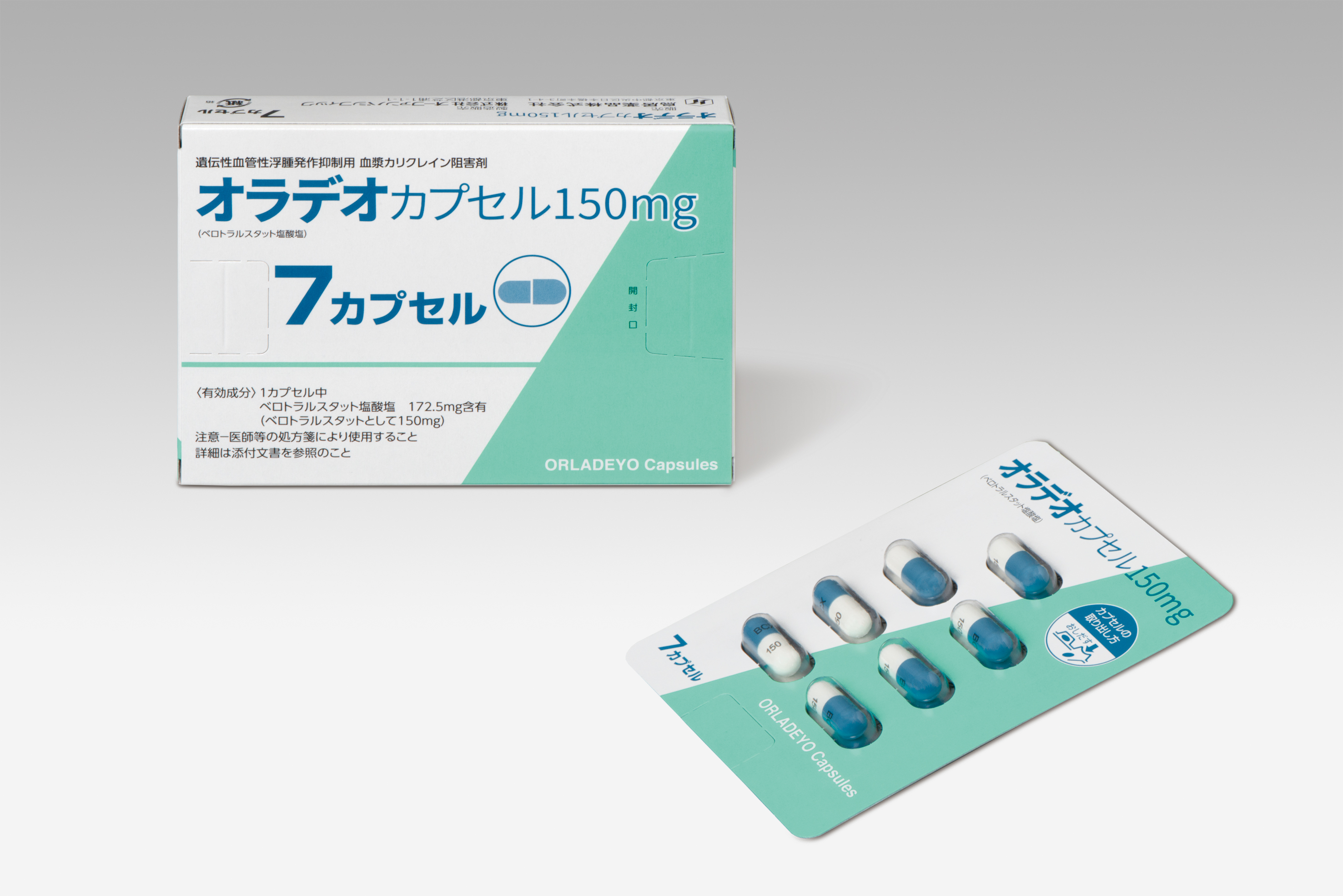
Continued here:
BioCryst Announces Approval of ORLADEYO™ (berotralstat) in Japan for the Prophylactic Treatment of Hereditary Angioedema
PCI Biotech to present at RNA Therapeutics Virtual Conference
By Dr. Matthew Watson
Oslo (Norway), 22 January 2021 – PCI Biotech (OSE: PCIB), a clinical-stage biopharma company developing innovative therapeutics that address significant unmet medical needs in cancer today announced that it will present at the 12th Annual RNA Therapeutics Virtual Conference, a UK based online event taking place February 10-11, 2021. The 2021 conference is set to explore the latest developments in RNA delivery agents and RNA-based therapeutics with the latest case studies on advanced mRNA technologies, oligonucleotide delivery, therapeutic applications and future trends and innovations. PCI Biotech is also a sponsor of the event.
Read the original here:
PCI Biotech to present at RNA Therapeutics Virtual Conference
Mydecine Innovations Group Included in First-Ever Psychedelics ETF
By Dr. Matthew Watson
DENVER, Jan. 22, 2021 (GLOBE NEWSWIRE) -- Mydecine Innovations Group (CSE: MYCO) (OTC: MYCOF) (FSE: 0NFA) (“Mydecine” or the “Company’), an emerging biopharma and life sciences company committed to the research, development, and acceptance of alternative nature-sourced medicine for mainstream use, has been included in the first-ever Psychedelics Exchanged Traded Fund (ETF).
Visit link:
Mydecine Innovations Group Included in First-Ever Psychedelics ETF
Equillium Announces Acceptance of Late-Breaking Abstract for Oral Presentation of Interim Data from EQUATE Study in acute GVHD at the 2021 TCT…
By Dr. Matthew Watson
LA JOLLA, Calif., Jan. 22, 2021 (GLOBE NEWSWIRE) -- Equillium, Inc. (Nasdaq: EQ) a clinical-stage biotechnology company developing itolizumab to treat severe autoimmune and inflammatory disorders, today announced that interim data from the Phase 1b/2 EQUATE study of itolizumab in acute graft-versus-host disease (aGVHD) has been accepted as a late-breaking oral presentation at the 2021 TCT Meetings Digital Experience, being held February 8-12, 2021.
Read the original here:
Equillium Announces Acceptance of Late-Breaking Abstract for Oral Presentation of Interim Data from EQUATE Study in acute GVHD at the 2021 TCT...


 January 23rd, 2021
January 23rd, 2021
