BioCryst to Present at JMP Securities Hematology Summit
By Dr. Matthew Watson
RESEARCH TRIANGLE PARK, N.C., Dec. 11, 2020 (GLOBE NEWSWIRE) -- BioCryst Pharmaceuticals, Inc. (Nasdaq: BCRX) today announced that the company will present at the JMP Securities Hematology Summit, which is being conducted as a virtual event, on Tuesday, December 15, 2020 at 3:30 p.m. ET.
See more here:
BioCryst to Present at JMP Securities Hematology Summit
New Study Provides Personalized Breast Cancer Risk Information for Women with ATM Gene Mutations
By Dr. Matthew Watson
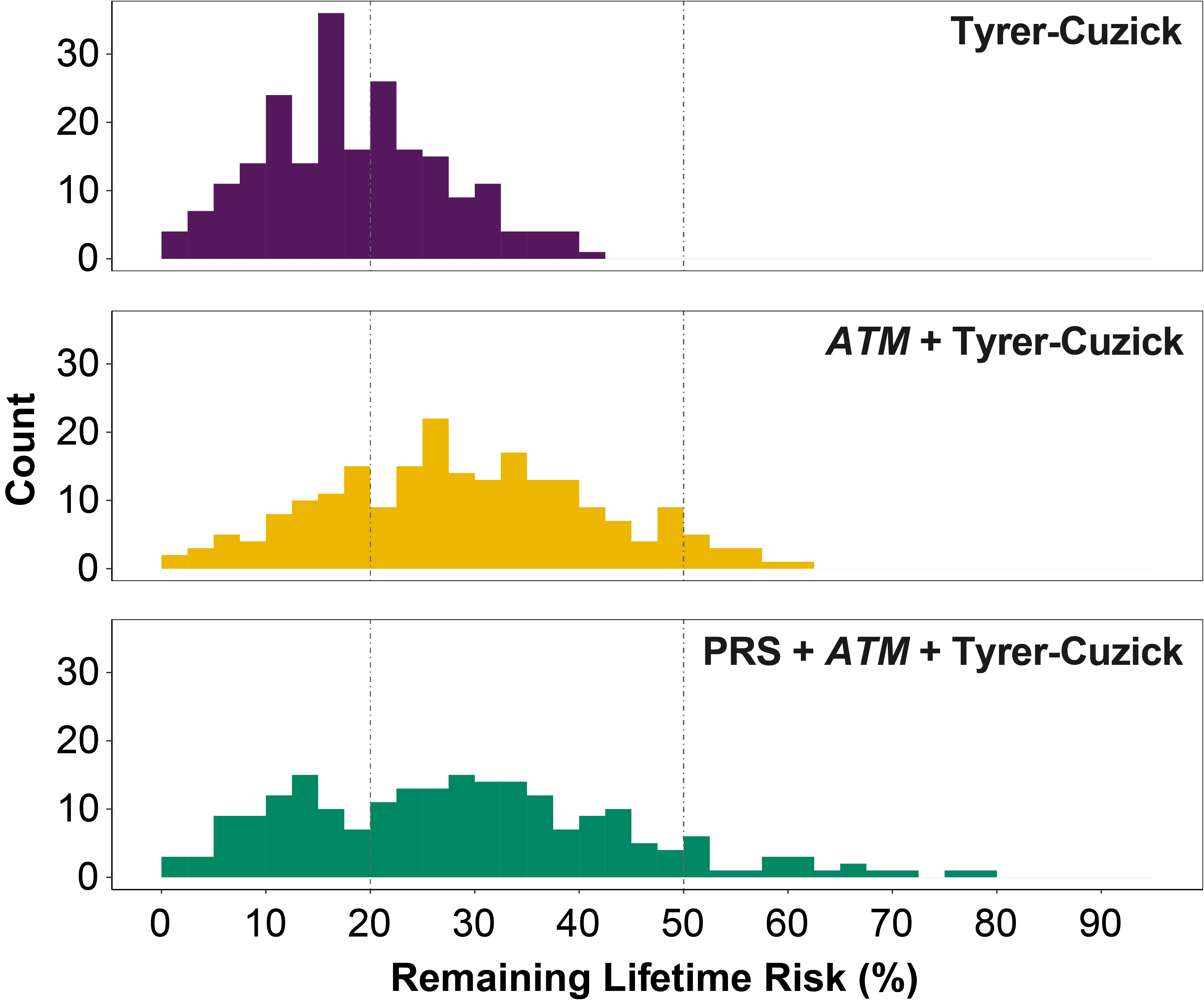
Here is the original post:
New Study Provides Personalized Breast Cancer Risk Information for Women with ATM Gene Mutations
Altimmune to Present at the 4th Annual NASH Summit 2020 Digital Conference
By Dr. Matthew Watson
GAITHERSBURG, Md., Dec. 11, 2020 (GLOBE NEWSWIRE) -- Altimmune, Inc. (Nasdaq: ALT), a clinical-stage biopharmaceutical company, today announced that Dr. Scott Harris, Chief Medical Officer of Altimmune will give an oral presentation on ALT-801, the Company’s novel GLP-1/Glucagon dual receptor agonist in development for the treatment of non-alcoholic steatohepatitis (NASH.) Dr. Harris will also participate on a panel discussion on NASH treatments. The presentations will take place at the 4th Annual NASH Summit 2020 Digital Conference, which is being held December 15-18, 2020.
Read more here:
Altimmune to Present at the 4th Annual NASH Summit 2020 Digital Conference
Arch Therapeutics to Present at the 13th Annual LD Micro Main Event Conference
By Dr. Matthew Watson
FRAMINGHAM, Mass., Dec. 11, 2020 (GLOBE NEWSWIRE) -- Arch Therapeutics, Inc. (OTCQB: ARTH) ("Arch" or the "Company"), developer of novel self-assembling wound care and biosurgical devices, announced today that it will be presenting at the 13th annual LD Micro Main Event investor conference. This investor conference will take place December 14-15, 2020, on the Sequire Virtual Events platform.
See original here:
Arch Therapeutics to Present at the 13th Annual LD Micro Main Event Conference
Lexicon Pharmaceuticals Receives Fast Track Designation From the FDA for LX9211 for Diabetic Peripheral Neuropathic Pain
By Dr. Matthew Watson
THE WOODLANDS, Texas, Dec. 11, 2020 (GLOBE NEWSWIRE) -- Lexicon Pharmaceuticals, Inc. (Nasdaq: LXRX), announced today that it has received Fast Track designation from the U.S. Food and Drug Administration (FDA) for the development of LX9211 in diabetic peripheral neuropathic pain.
See the rest here:
Lexicon Pharmaceuticals Receives Fast Track Designation From the FDA for LX9211 for Diabetic Peripheral Neuropathic Pain
Cynata Secures $15m Placement Led By $10m from Healthcare Investor BioScience Managers to Expand Development Pipeline
By Dr. Matthew Watson
MELBOURNE, Australia, Dec. 11, 2020 (GLOBE NEWSWIRE) -- Cynata Therapeutics Limited (ASX: “CYP”, “Cynata”, or the “Company”), a clinical-stage biotechnology company specialising in cell therapeutics, is pleased to announce a successful $15 million placement (“Placement”), led by a $10m investment from experienced healthcare investor BioScience Managers through the BioScience Managers Translation Fund I (BMTFI). BMTFI has a mandate to invest in Australian based innovative healthcare technology and this investment will allow Cynata to significantly expand its clinical development pipeline and scale their operations in Australia. The Placement is being undertaken at an offer price of $0.70 for each new share and will be followed by a 1 for 15 non-renounceable entitlement offer at the same offer price as the Placement.
Go here to see the original:
Cynata Secures $15m Placement Led By $10m from Healthcare Investor BioScience Managers to Expand Development Pipeline
ExCellThera receives Priority Medicines (PRIME) designation from European Medicines Agency for ECT-001 Cell Therapy
By Dr. Matthew Watson
MONTREAL, Dec. 11, 2020 (GLOBE NEWSWIRE) -- ExCellThera Inc., a clinical-stage molecular medicine company delivering molecules and bioengineering solutions to expand stem and immune cells for therapeutic use, announced today that ECT-001 Cell Therapy has been granted PRIority MEdicines (PRIME) designation by the European Medicines Agency (EMA) for use in urgent allogeneic haematopoietic stem cell transplants.
The rest is here:
ExCellThera receives Priority Medicines (PRIME) designation from European Medicines Agency for ECT-001 Cell Therapy
Financial calendar for Zealand Pharma in 2021
By Dr. Matthew Watson
Company announcement – No. 59 / 2020
See the rest here:
Financial calendar for Zealand Pharma in 2021
Galecto Reports Third Quarter 2020 Operating & Financial Results and Provides a Corporate Update
By Dr. Matthew Watson
Successfully completed listing on US Nasdaq and raised over $150 million during recent IPO and preceding crossover round
See the article here:
Galecto Reports Third Quarter 2020 Operating & Financial Results and Provides a Corporate Update
Rhythm Pharmaceuticals Announces Appointments of Camille L. Bedrosian, M.D., and Lynn Tetrault, J.D., to its Board of Directors
By Dr. Matthew Watson
BOSTON, Dec. 11, 2020 (GLOBE NEWSWIRE) -- Rhythm Pharmaceuticals, Inc. (Nasdaq:RYTM), a biopharmaceutical company aimed at developing and commercializing therapies for the treatment of rare genetic diseases of obesity, today announced the appointments of Camille L. Bedrosian, M.D., and Lynn Tetrault, J.D., to its Board of Directors.
SELLAS Announces Positive Follow-up Data from the Randomized Phase 2 VADIS Trial of Nelipepimut-S (NPS) in Women with Ductal Carcinoma In-Situ of the…
By Dr. Matthew Watson
– Immune Stimulation Augmented by +1,300% at 6-months Post-NPS Treatment –
Soleno Therapeutics Provides Regulatory Update on DCCR for the Treatment of Prader-Willi Syndrome
By Dr. Matthew Watson
Soleno intends to submit plans to FDA to conduct further analyses of clinical data from completed and ongoing studies of DCCR, together with external, natural history studies Soleno intends to submit plans to FDA to conduct further analyses of clinical data from completed and ongoing studies of DCCR, together with external, natural history studies
Read more here:
Soleno Therapeutics Provides Regulatory Update on DCCR for the Treatment of Prader-Willi Syndrome
New sales reporting structure introduced. 2020 organic sales growth outlook now expected at 0%, from previously -2% to +2%
By Dr. Matthew Watson
Please read the full announcement in PDF
See the article here:
New sales reporting structure introduced. 2020 organic sales growth outlook now expected at 0%, from previously -2% to +2%
Akari Therapeutics Announces New Clinical Data that Show Long-Term Self-Administered Nomacopan is Well-Tolerated and Substantially Reduces Transfusion…
By Dr. Matthew Watson
NEW YORK and LONDON, Dec. 11, 2020 (GLOBE NEWSWIRE) -- Akari Therapeutics, Plc (Nasdaq: AKTX), a late-stage biopharmaceutical company focused on innovative therapeutics to treat orphan autoimmune and inflammatory diseases where the complement and/or leukotriene systems are implicated, announces new data on the efficacy and safety profile of long-term self-administration of nomacopan for treatment of patients with PNH.
Go here to read the rest:
Akari Therapeutics Announces New Clinical Data that Show Long-Term Self-Administered Nomacopan is Well-Tolerated and Substantially Reduces Transfusion...
CHMP recommends approval of Plavix® (clopidogrel) with aspirin in adults for certain types of strokes
By Dr. Matthew Watson
CHMP recommends approval of Plavix® (clopidogrel) with aspirin in adults for certain types of strokes
See original here:
CHMP recommends approval of Plavix® (clopidogrel) with aspirin in adults for certain types of strokes
Akari Therapeutics Reports Third Quarter 2020 Financial Results and Highlights Recent Clinical Progress
By Dr. Matthew Watson
NEW YORK and LONDON, Dec. 11, 2020 (GLOBE NEWSWIRE) -- Akari Therapeutics, Plc (Nasdaq: AKTX), a late-stage biopharmaceutical company focused on innovative therapeutics to treat orphan autoimmune and inflammatory diseases where complement (C5) and/or leukotriene (LTB4) systems are implicated, today announced financial results for the third quarter ended September 30, 2020, as well as recent clinical progress.
Read more from the original source:
Akari Therapeutics Reports Third Quarter 2020 Financial Results and Highlights Recent Clinical Progress
AskBio CSO Jude Samulski Named 2020 Business Person of the Year by Triangle Business Journal
By Dr. Matthew Watson
RESEARCH TRIANGLE PARK, N.C., Dec. 11, 2020 (GLOBE NEWSWIRE) -- Asklepios BioPharmaceutical, Inc. (AskBio), a leading, clinical-stage adeno-associated virus (AAV) gene therapy company, today announced that its President and Chief Scientific Officer, Jude Samulski, PhD, has been honored as Triangle Business Journal’s (TBJ) 2020 Business Person of the Year.
See the original post here:
AskBio CSO Jude Samulski Named 2020 Business Person of the Year by Triangle Business Journal
Tauriga Sciences Inc. Commences Development of Vegan, CBD Infused Pet Treat
By Dr. Matthew Watson
NEW YORK, NY, Dec. 11, 2020 (GLOBE NEWSWIRE) -- via NewMediaWire -- Tauriga Sciences, Inc. (OTCQB: TAUG) (“Tauriga” or the “Company”), a revenue generating, diversified life sciences company, with a proprietary line of functional “supplement” chewing gums (Flavors: Pomegranate, Blood Orange, Peach-Lemon, Pear Bellini, Mint, Black Currant) as well as two ongoing Biotechnology initiatives, today announced that it has commenced the development of a Vegan, Cannabidiol (“CBD”) infused Pet Food Treat (“Pet Treat”). The Company has contracted an industry expert to help with: formulations, specifications, designs, regulations, and certifications. The Company’s aim is to commercially launch its proposed Pet Treat product, during March 2021.
Read more from the original source:
Tauriga Sciences Inc. Commences Development of Vegan, CBD Infused Pet Treat
Exodus Effect Review – CBD Oil launched – Product Review by Mike Vaughn
By Dr. Matthew Watson
Exodus Effect CBD Oil is made of 100% natural ingredients that are specially formulated to alleviate pain. Find out yourself in our review. Exodus Effect CBD Oil is made of 100% natural ingredients that are specially formulated to alleviate pain. Find out yourself in our review.
Read the original:
Exodus Effect Review - CBD Oil launched – Product Review by Mike Vaughn
Cronos Group Inc. to Present at the MKM Partners Virtual Conference
By Dr. Matthew Watson
TORONTO, Dec. 11, 2020 (GLOBE NEWSWIRE) -- Cronos Group Inc. (NASDAQ: CRON) (TSX: CRON) (“Cronos Group” or the “Company”), an innovative global cannabinoid company, today announced that Mike Gorenstein, Executive Chairman, will present at The Road Ahead, Preparation for 2021: MKM Partners Virtual Conference on Wednesday, December 16, 2020 at 12:30 p.m. EST.
Read more:
Cronos Group Inc. to Present at the MKM Partners Virtual Conference


 December 11th, 2020
December 11th, 2020