Making it easier to make stem cells: Kinase inhibitors lower barrier to producing stem cells in lab
By daniellenierenberg
ScienceDaily (Sep. 25, 2012) The process researchers use to generate induced pluripotent stem cells (iPSCs) -- a special type of stem cell that can be made in the lab from any type of adult cell -- is time consuming and inefficient. To speed things up, researchers at Sanford-Burnham Medical Research Institute (Sanford-Burnham) turned to kinase inhibitors. These chemical compounds block the activity of kinases, enzymes responsible for many aspects of cellular communication, survival, and growth.
As they outline in a paper published September 25 in Nature Communications, the team found several kinase inhibitors that, when added to starter cells, help generate many more iPSCs than the standard method. This new capability will likely speed up research in many fields, better enabling scientists around the world to study human disease and develop new treatments.
"Generating iPSCs depends on the regulation of communication networks within cells," explained Tariq Rana, Ph.D., program director in Sanford-Burnham's Sanford Children's Health Research Center and senior author of the study. "So, when you start manipulating which genes are turned on or off in cells to create pluripotent stem cells, you are probably activating a large number of kinases. Since many of these active kinases are likely inhibiting the conversion to iPSCs, it made sense to us that adding inhibitors might lower the barrier."
According to Tony Hunter, Ph.D., professor in the Molecular and Cell Biology Laboratory at the Salk Institute for Biological Studies and director of the Salk Institute Cancer Center, "The identification of small molecules that improve the efficiency of generating iPSCs is an important step forward in being able to use these cells therapeutically. Tariq Rana's exciting new work has uncovered a class of protein kinase inhibitors that override the normal barriers to efficient iPSC formation, and these inhibitors should prove useful in generating iPSCs from new sources for experimental and ultimately therapeutic purposes." Hunter, a kinase expert, was not involved in this study.
The promise of iPSCs
At the moment, the only treatment option available to many heart failure patients is a heart transplant. Looking for a better alternative, many researchers are coaxing stem cells into new heart muscle. In Alzheimer's disease, researchers are also interested in stem cells, using them to reproduce a person's own malfunctioning brain cells in a dish, where they can be used to test therapeutic drugs. But where do these stem cells come from? Since the advent of iPSC technology, the answer in many cases is the lab. Like their embryonic cousins, iPSCs can be used to generate just about any cell type -- heart, brain, or muscle, to name a few -- that can be used to test new therapies or potentially to replace diseased or damaged tissue.
It sounds simple enough: you start with any type of differentiated cell, such as skin cells, add four molecules that reprogram the cells' genomes, and then try to catch those that successfully revert to unspecialized iPSCs. But the process takes a long time and isn't very efficient -- you can start with thousands of skin cells and end up with just a few iPSCs.
Inhibiting kinases to make more iPSCs
Zhonghan Li, a graduate student in Rana's laboratory, took on the task of finding kinase inhibitors that might speed up the iPSC-generating process. Scientists in the Conrad Prebys Center for Chemical Genomics, Sanford-Burnham's drug discovery facility, provided Li with a collection of more than 240 chemical compounds that inhibit kinases. Li painstakingly added them one-by-one to his cells and waited to see what happened. Several kinase inhibitors produced many more iPSCs than the untreated cells -- in some cases too many iPSCs for the tiny dish housing them. The most potent inhibitors targeted three kinases in particular: AurkA, P38, and IP3K.
Working with the staff in Sanford-Burnham's genomics, bioinformatics, animal modeling, and histology core facilities -- valuable resources and expertise available to all Sanford-Burnham scientists and the scientific community at large -- Rana and Li further confirmed the specificity of their findings and even nailed down the mechanism behind one inhibitor's beneficial actions.
See the original post:
Making it easier to make stem cells: Kinase inhibitors lower barrier to producing stem cells in lab
Making it easier to make stem cells
By Dr. Matthew Watson
Public release date: 25-Sep-2012 [ | E-mail | Share ]
Contact: Heather Buschman hbuschman@sanfordburnham.org 858-795-5343 Sanford-Burnham Medical Research Institute
LA JOLLA, Calif., September 25, 2012 The process researchers use to generate induced pluripotent stem cells (iPSCs)a special type of stem cell that can be made in the lab from any type of adult cellis time consuming and inefficient. To speed things up, researchers at Sanford-Burnham Medical Research Institute (Sanford-Burnham) turned to kinase inhibitors. These chemical compounds block the activity of kinases, enzymes responsible for many aspects of cellular communication, survival, and growth. As they outline in a paper published September 25 in Nature Communications, the team found several kinase inhibitors that, when added to starter cells, help generate many more iPSCs than the standard method. This new capability will likely speed up research in many fields, better enabling scientists around the world to study human disease and develop new treatments.
"Generating iPSCs depends on the regulation of communication networks within cells," explained Tariq Rana, Ph.D., program director in Sanford-Burnham's Sanford Children's Health Research Center and senior author of the study. "So, when you start manipulating which genes are turned on or off in cells to create pluripotent stem cells, you are probably activating a large number of kinases. Since many of these active kinases are likely inhibiting the conversion to iPSCs, it made sense to us that adding inhibitors might lower the barrier."
According to Tony Hunter, Ph.D., professor in the Molecular and Cell Biology Laboratory at the Salk Institute for Biological Studies and director of the Salk Institute Cancer Center, "The identification of small molecules that improve the efficiency of generating iPSCs is an important step forward in being able to use these cells therapeutically. Tariq Rana's exciting new work has uncovered a class of protein kinase inhibitors that override the normal barriers to efficient iPSC formation, and these inhibitors should prove useful in generating iPSCs from new sources for experimental and ultimately therapeutic purposes." Hunter, a kinase expert, was not involved in this study.
The promise of iPSCs
At the moment, the only treatment option available to many heart failure patients is a heart transplant. Looking for a better alternative, many researchers are coaxing stem cells into new heart muscle. In Alzheimer's disease, researchers are also interested in stem cells, using them to reproduce a person's own malfunctioning brain cells in a dish, where they can be used to test therapeutic drugs. But where do these stem cells come from? Since the advent of iPSC technology, the answer in many cases is the lab. Like their embryonic cousins, iPSCs can be used to generate just about any cell typeheart, brain, or muscle, to name a fewthat can be used to test new therapies or potentially to replace diseased or damaged tissue.
It sounds simple enough: you start with any type of differentiated cell, such as skin cells, add four molecules that reprogram the cells' genomes, and then try to catch those that successfully revert to unspecialized iPSCs. But the process takes a long time and isn't very efficientyou can start with thousands of skin cells and end up with just a few iPSCs.
Inhibiting kinases to make more iPSCs
Zhonghan Li, a graduate student in Rana's laboratory, took on the task of finding kinase inhibitors that might speed up the iPSC-generating process. Scientists in the Conrad Prebys Center for Chemical Genomics, Sanford-Burnham's drug discovery facility, provided Li with a collection of more than 240 chemical compounds that inhibit kinases. Li painstakingly added them one-by-one to his cells and waited to see what happened. Several kinase inhibitors produced many more iPSCs than the untreated cellsin some cases too many iPSCs for the tiny dish housing them. The most potent inhibitors targeted three kinases in particular: AurkA, P38, and IP3K.
See more here:
Making it easier to make stem cells
Eastday-Shanghai doctors reveal face-change leap
By daniellenierenberg
SHANGHAI doctors announced the success of a novel technology that uses people's own skin via stem cells to grow a new face for seriously disfigured patients.
It's an alternative to the surgery used in the West in which doctors transplant the face from a dead body to a patient.
Facial tissue developed with the new technology is more readily accepted physically and psychologically by patients and has no ethical issues, doctors from Shanghai No. 9 People's Hospital said yesterday.
Since adopting the new technology, doctors have used it on more than 60 patients, including seven who needed their whole face replaced or major facial changes.
Of the seven, six were a success, while one case failed as skin on part of the face died, doctors said.
Patients include women disfigured by having sulfuric acid splashed in their faces, people who lost their nose during a fight and a person whose face was seriously burned in a fire.
Under the technology, doctors remove certain blood vessels from the patient's leg to build a small vessel net and transplant it into a place on the body to grow the new face, usually on the a patient's upper chest.
Then doctors use a skin dilator to expand the skin like a bulging ball. Later they inject the patient's own stem cells to help the skin grow stronger and stimulate the growth of blood vessels.
Soft bones which are shaped into facial features like a nose and upper jaw bone in line with the patient's own facial skeleton are then transplanted under the new facial skin.
Finally, the new face is transplanted onto the disfigured face. The new face, which is thin and comprised of a whole piece of living skin, will join with the facial muscles, thus giving a patient natural facial expressions and function to the greatest extent possible.
Introducing Canadians to a whole new way to treat aging skin: Stemulation
By Dr. Matthew Watson
TORONTO, Sept. 10, 2012 /CNW/ - Sigmacon Skin Sciences announced it is the exclusive Canadian distributor of Stemulation, a luxury skin care line that uses the healing power of human stem cells to combat wrinkles and other signs of aging.
Stemulation is based on the science that stem cells can be effectively used for skin rejuvenation, tissue repair and wound healing. A research team of specialists spent two years capturing growth factors from adult human skin cells, which they turned into an active ingredient and the basis for Stemulation products. These growth factors stimulate collagen and the reproduction of new skin cells to reduce wrinkles, eliminate sun spots and smooth scars and fine lines. It truly is a groundbreaking (and technology-backed) new way to achieve younger-looking skin!
The Stemulation line includes a serum, cleanser, exfoliant and face and body creams. The line will be sold through select doctors, estheticians and medical spas.
ABOUT Sigmacon Skin Sciences is the national distributor of a comprehensive set of performance skin care products with dedicated product specialists and trainings all across Canada. Our product lines include professional treatments, sun protection products and results-oriented home care. Sigmacon is also the distributor of advanced medical and aesthetic devices. Visit http://www.skinsciences.ca to learn more.
Image with caption: "The Future of Skin Care: Stemulation Facial Serum and Boost Crme used over 1 year. (CNW Group/Sigmacon Skin Sciences)". Image available at: http://photos.newswire.ca/images/download/20120910_C3135_PHOTO_EN_17420.jpg
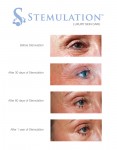
The rest is here:
Introducing Canadians to a whole new way to treat aging skin: Stemulation
In real time, Yale scientists watch stem cells at work regenerating tissue
By LizaAVILA
Scientists have for the first time watched and manipulated stem cells as they regenerate tissue in an uninjured mammal, Yale researchers report July 1 online in the journal Nature.
Using a sophisticated imaging technique, the researchers also demonstrated that mice lacking a certain type of cell do not regrow hair. The same technique could shed light on how stem cells interact with other cells and trigger repairs in a variety of other organs, including lung and heart tissue.
This tells us a lot about how the tissue regeneration process works, said Valentina Greco, assistant professor of genetics and of dermatology at the Yale Stem Cell Center, researcher for the Yale Cancer Center and senior author of the study.
Greco and her team focused on stem cell behavior in the hair follicle of the mouse. The accessibility of the hair follicle allowed real-time and non-invasive imaging through a technology called 2-photon intravital microscopy.
Using this method, Panteleimon Rompolas, a post-doctoral fellow in Grecos lab and lead author of this paper, was able to study the interaction between stem cells and their progeny, which produce all the different types of cells in the tissue. The interaction of these cells with the immediate environment determines how cells divide, where they migrate and which specialized cells they become.
The technology allowed the team to discover that hair growth in mice cannot take place in the absence of connective tissue called mesenchyme, which appears early in embryonic development.
Stem cells not only spur growth of hair in mammals including humans, but also can serve to regenerate many other types of tissues.
Understanding how stem cell behavior is regulated by the microenvironment can advance our use of stem cells for therapeutic purposes and uncover mechanisms that go wrong in cancer and other diseases, Greco said.
The study was funded by an Alexander Brown Coxe postdoctoral fellowship. This work was supported in part by the American Skin Association and the American Cancer Society and the Yale Rheumatologic Disease Research Core Center and the National Institutes of Health.
Other Yale authors include Elizabeth Deschene, Giovanni Zito, David G. Gonzalez, Ichiko Saotome and Ann M. Haberman.
Go here to read the rest:
In real time, Yale scientists watch stem cells at work regenerating tissue
Brain Cells Derived From Skin Cells For Huntington's Research
By Dr. Matthew Watson
Editor's Choice Main Category: Huntingtons Disease Also Included In: Stem Cell Research;Neurology / Neuroscience Article Date: 29 Jun 2012 - 14:00 PDT
Current ratings for: Brain Cells Derived From Skin Cells For Huntington's Research
3 (1 votes)
At present, there is no cure for the disease and no treatments are available. These findings open up the possibility of testing treatments for the deadly disorder in a petri dish.
The study is the work of a Huntington's Disease iPSC Consortium, including researchers from the Johns Hopkins University School of Medicine in Baltimore, Cedars-Sinai Medical Center in Los Angeles and the University of California, Irvine, and six other groups.
Huntington's disease is an inherited, deadly neurodegenerative disorder. The onset of HD generally occurs during midlife, although it can also strike in childhood - as in the patient who donated the material for the cells generated in this study. The disease causes jerky, twitch-like movements, lack of muscle control, psychiatric disorders and dementia, and ultimately death.
Christopher A. Ross, M.D., Ph.D., a professor of psychiatry and behavioral sciences, neurology, pharmacology and neuroscience at the Johns Hopkins University School of Medicine and one of the lead researchers of the study, explained:
The team are currently testing small molecules for the ability to block HP iPSC degeneration. According to the researchers, these molecules could potentially be developed into new drugs for Huntington's disease.
Furthermore, the teams ability to create "HD in a dish" may also have implications for similar research in other diseases such as Parkinson's and Alzheimer's.
In the study, the team took a skin biopsy from a 7-year-old patient with very early onset of severe HD. In the laboratory of Hongjun Song, Ph.D., a professor at Johns Hopkins' Institute for Cell Engineering, the skin cells were grown in culture and then created into pluripotent stem cells. In addition, a second cell line was created in the same way in Dr. Ross's lab from an individuals without HD.Simultaneously, other HD and control iPS cell lines were generated as part of the NINDS funded HD iPS cell consortium.
Read this article:
Brain Cells Derived From Skin Cells For Huntington's Research
Skin Cells Create Stem Cells In Huntington Disease Study
By Dr. Matthew Watson
June 29, 2012
Connie K. Ho for redOrbit.com Your Universe Online
In 1993, the autosomal dominant gene mutation responsible for Huntingtons Disease (HD) was discovered. However, no treatments are known to slow its progression. New research may pave the way to better understanding of the disease. Researchers at Johns Hopkins recently announced that they were able to produce stem cells from skin cells from a person who had severe, early-onset form of HD; the cells were then changed into neurons that degenerated like the cells affected by HD.
The research was recently published in the journal Cell Stem Cell. The investigators worked with an international consortium in creating HD in a dish. The group was made up of scientists from Johns Hopkins University School of Medicine, Cedars-Sinai Medical Center, the University of California at Irvine, as well as six other groups. The team looked at many other HD cell lines and control cell lines to verify that the results were consistent and reproducible in other labs. The investigators believe that the findings allow them to better understand and eliminate cells in people in with HD. They hope to study the effects of possible drug treatments on cells that would be otherwise found deep in the brain.
Having these cells will allow us to screen for therapeutics in a way we havent been able to before in Huntingtons disease, remarked lead researcher Dr. Christopher A. Ross, a professor of psychiatry and behavioral sciences, neurology, pharmacology and neuroscience at the Johns Hopkins University School of Medicine, in a prepared statement. For the first time, we will be able to study how drugs work on human HD neurons and hopefully take those findings directly to the clinic.
The team of researchers is studying small molecules for the ability to block HD iPSC degeneration to see if they can be developed into new drugs for HD. As well, the ability to produce from stem cells the same neurons found in HD may have effects for similar research in other neurodegenerative diseases like Alzheimers and Parkinsons. In the experiment, Ross took a skin biopsy from a patient with very early onset HD. The patient was seven years old at the time, with a severe form of disease and a mutation that caused it. By using cells from a patient who had quickly progressing HD, Ross team were able to mimic HD in a way that could be used by patients who had different forms of HD.
The skin cells were grown in culture and reprogrammed to induce stem cells that were pluripotent. Then, another cell line was created in the same way from someone who didnt have HD. The other HD and control iPS cells were produced as part of the NINDS funded HD iPS cell consortium. Investigators from Johns Hopkins and the other consortium labs changed the cells into typical neurons and then into medium spiny neurons. The process took a total of three months and the scientists found the medium spiny neurons from the HD cells acted how the medium spiny neurons form an HD patient would. The cells demonstrated quick degeneration when cultured in the lab with a basic culture medium that didnt include extensive supporting nutrients. On the other hand, control cell lines didnt demonstrate neuronal degeneration.
These HD cells acted just as we were hoping, says Ross, director of the Baltimore Huntingtons Disease Center. A lot of people said, Youll never be able to get a model in a dish of a human neurodegenerative disease like this. Now, we have them where we can really study and manipulate them, and try to cure them of this horrible disease. The fact that we are able to do this at all still amazes us.
Source: Connie K. Ho for redOrbit.com Your Universe Online
Here is the original post:
Skin Cells Create Stem Cells In Huntington Disease Study
Huntington’s disease neurons created from stem cells
By raymumme
An international consortium of Huntington's disease experts, including several from the Sue & Bill Gross Stem Cell Research Center at UC Irvine and the UCSF Gladstone Institutes, has generated a human model of the deadly inherited disorder directly from the skin cells of affected patients.
The re-created neurons, which live in a petri dish, will help researchers better understand what disables and kills brain cells in people with HD and let them gauge the effects of potential drug therapies on cells that are otherwise locked deep in the brain.
UCI scientists were part of a consortium that in 1993 identified the autosomal dominant gene mutation responsible for HD, but there is still no cure, and no treatments are available to even slow its onset or progression. The research, published online today in the journal Cell Stem Cell, is the work of the Huntington's Disease iPSC Consortium. Participants examined several other cell lines and control cell lines to ensure that their results were consistent and reproducible in different labs.
"Our discovery will enable us for the first time to test therapies on human Huntington's disease neurons," said Leslie Thompson, UCI professor of psychiatry & human behavior and neurobiology & behavior, one of the world's leading HD experts and a senior author of the study. "This has been a remarkable time in HD research, with the advent of stem cell technologies that have allowed these scientific advancements. Also, having a team of scientists working together as a consortium has benefited the research tremendously and accelerated its pace."
Huntington's is such a rare disease, although it is the most common inherited neurodegenerative disorder. It afflicts approximately 30,000 people in the United States-with another 75,000 people carrying the gene that will eventually lead to it.
"An advantage of this human model is that we now have the ability to identify changes in brain cells over time-during the degeneration process and at specific stages of brain-cell development," said Gladstone Senior Investigator Dr. Steve Finkbeiner. "We hope this model will help us more readily uncover relevant factors that contribute to Huntington's disease and especially to find successful therapeutic approaches."
UC Irvine press release
Gladstone Institutes press release
Read more:
Huntington’s disease neurons created from stem cells
Scientists Correct Huntington's Mutation in Induced Pluripotent Stem Cells
By NEVAGiles23
Newswise Researchers at the Buck Institute have corrected the genetic mutation responsible for Huntingtons Disease (HD) using a human induced pluripotent stem cell (iPSC) that came from a patient suffering from the incurable, inherited neurodegenerative disorder. Scientists took the diseased iPSCs, made the genetic correction, generated neural stem cells and then transplanted the mutation-free cells into a mouse model of HD where they are generating normal neurons in the area of the brain affected by HD. Results of the research are published in the June 28, 2012 online edition of the journal Cell Stem Cell.
iPSCs are reverse-engineered from human cells such as skin, back to a state where they can be coaxed into becoming any type of cell. They can be used to model numerous human diseases and may also serve as sources of transplantable cells that can be used in novel cell therapies. In the latter case, the patient provides a sample of his or her own skin to the laboratory. We believe the ability to make patient-specific, genetically corrected iPSCs from HD patients is a critical step for the eventual use of these cells in cell replacement therapy, said Buck faculty Lisa Ellerby, PhD, lead author of the study. The genetic correction reversed the signs of disease in these cells the neural stem cells were no longer susceptible to cell death and the function of their mitochondria was normal. Ellerby said the corrected cells could populate the area of the mouse brain affected in HD, therefore, the next stage of research involves transplantation of corrected cells to see if the HD-afflicted mice show improved function. Ellerby said these studies are important as now we can deliver patient-specific cells for cell therapy, that no longer have the disease causing mutation.
Huntington's disease (HD) is a devastating, neurodegenerative genetic disorder that affects muscle coordination and leads to cognitive decline and psychiatric problems. It typically becomes noticeable in mid-adult life, with symptoms beginning between 35 and 44 years of age. Life expectancy following onset of visual symptoms is about 20 years. The worldwide prevalence of HD is 5-10 cases per 100,000 persons. More than a quarter of a million Americans have HD or are "at risk" of inheriting the disease from an affected parent. Key to the disease process is the formation of specific protein aggregates (essentially abnormal clumps) inside some neurons.
All humans have two copies of the Huntingtin gene (HTT), which codes for the protein Huntingtin (Htt). Part of this gene is a repeated section called a trinucleotide repeat, which varies in length between individuals and may change between generations. When the length of this repeated section reaches a certain threshold, it produces an altered form of the protein, called mutant Huntingtin protein (mHtt). Scientists in the Ellerby lab corrected the mutation by replacing the expanded trinucleotide repeat with a normal repeat using homologous recombination. Homologous recombination is a type of genetic recombination where two molecules of DNA are exchanged. In this case the diseased DNA sequence is exchanged for the normal DNA sequence.
Contributors to the work: Mahru An and Ningzhe Zhang are shared first authors of this study. Other Buck Institute researchers involved in the study include Gary Scott, Daniel Montoro, Tobias Wittkop, and faculty members Sean Mooney and Simon Melov. The work was funded by the Buck Institute and the National Institutes of Health.
About the Buck Institute for Research on Aging The Buck Institute is the U.S.s first and foremost independent research organization devoted to Geroscience focused on the connection between normal aging and chronic disease. Based in Novato, CA, The Buck is dedicated to extending Healthspan, the healthy years of human life and does so utilizing a unique interdisciplinary approach involving laboratories studying the mechanisms of aging and those focused on specific diseases. Buck scientists strive to discover new ways of detecting, preventing and treating age-related diseases such as Alzheimers and Parkinsons, cancer, cardiovascular disease, macular degeneration, diabetes and stroke. In their collaborative research, they are supported by the most recent developments in genomics, proteomics and bioinformatics. For more information: http://www.thebuck.org.
Read more:
Scientists Correct Huntington's Mutation in Induced Pluripotent Stem Cells
Huntington's Research Tool Developed Using Stem Cells
By LizaAVILA
Main Category: Huntingtons Disease Also Included In: Stem Cell Research Article Date: 28 Jun 2012 - 9:00 PDT
Current ratings for: Huntington's Research Tool Developed Using Stem Cells
Cedars-Sinai scientists have joined with expert colleagues around the globe in using stem cells to develop a laboratory model for Huntington's disease, allowing researchers for the first time to test directly on human cells potential treatments for this fatal, inherited disorder.
As explained in a paper published June 28 on the Cell Stem Cell website and scheduled for print in the journal's Aug. 3 issue, scientists at Cedars-Sinai's Regenerative Medicine Institute and the University of Wisconsin took skin cells from patients with Huntington's disease and reprogrammed them into powerful stem cells; these were then made into the nervous system cells affected by the disease. Seven laboratories around the world collaborated to demonstrate the cells had hallmarks of Huntington's.
"This Huntington's 'disease in a dish' will enable us for the first time to test therapies on human Huntington's disease neurons," said Clive Svendsen, PhD, director of the Cedars-Sinai Regenerative Medicine Institute and a senior author of the study. "In addition to increasing our understanding of this disorder and offering a new pathway to identifying treatments, this study is remarkable because of the extensive interactions between a large group of scientists focused on developing this model. It's a new way of doing trailblazing science."
The Huntington's Disease iPSC Consortium united some of the world's top scientists working on this disease. Cedars-Sinai researchers took skin cells from a several Huntington's patients, including a six-year-old with a severe juvenile form of the disease. They genetically reprogrammed these tissues into induced pluripotent stem cells, which can be made into any type of cell in the body. The cells lines were banked by scientists at Cedars-Sinai and scrutinized by all consortium members for differences that may have led to the disease. These cell lines are now an important resource for Huntington's researchers and have been made available via a National Institutes of Health-funded repository at Coriell Institute for Medical Research in New Jersey.
Huntington's, known to the public, for example, as the cause of folksinger Woody Guthrie's death, typically strikes patients in midlife. It causes jerky, twitching motions, loss of muscle control, psychiatric disorders and dementia; the disease ultimately is fatal. In rare, severe cases, the disorder appears in childhood.
Researchers believe that Huntington's results from a mutation in the huntintin gene, leading to production of an abnormal protein and ultimately cell death in specific areas of the brain that control movement and cognition. There is no cure for Huntington's, nor therapies to slow its progression.
The consortium showed Huntington's cell deficits or how they differ from normal cells, including that they were less likely to survive cultivation in the petri dish. Scientists tried depriving them of a growth factor present around normal cells, or "stressing" them, and found that Huntington's neurons died even faster.
"It was great that these characteristics were seen not only in our laboratory, but by all of the consortium members using different techniques," said Virginia Mattis, a post-doctoral scientist at the Cedars-Sinai Regenerative Medicine Institute and one of the lead authors of the study. "It was very reassuring and significantly strengthens the value of this study."
Read the rest here:
Huntington's Research Tool Developed Using Stem Cells
Human model of Huntington's disease created from skin's stem cells
By daniellenierenberg
Public release date: 28-Jun-2012 [ | E-mail | Share ]
Contact: Tom Vasich tmvasich@uci.edu 949-824-6455 University of California - Irvine
Irvine, Calif., June 28, 2012 An international consortium of Huntington's disease experts, including several from the Sue & Bill Gross Stem Cell Research Center at UC Irvine, has generated a human model of the deadly inherited disorder directly from the skin cells of affected patients.
The re-created neurons, which live in a petri dish, will help researchers better understand what disables and kills brain cells in people with HD and let them gauge the effects of potential drug therapies on cells that are otherwise locked deep in the brain.
UCI scientists were part of a consortium that in 1993 identified the autosomal dominant gene mutation responsible for HD, but there is still no cure, and no treatments are available to even slow its onset or progression. The research, published online today in the journal Cell Stem Cell, is the work of the Huntington's Disease iPSC Consortium. Participants examined several other cell lines and control cell lines to ensure that their results were consistent and reproducible in different labs.
"Our discovery will enable us for the first time to test therapies on human Huntington's disease neurons," said Leslie Thompson, UCI professor of psychiatry & human behavior and neurobiology & behavior, one of the world's leading HD experts and a senior author of the study. "This has been a remarkable time in HD research, with the advent of stem cell technologies that have allowed these scientific advancements. Also, having a team of scientists working together as a consortium has benefited the research tremendously and accelerated its pace."
Leslie Lock, a UCI assistant professor of developmental & cell biology and biological chemistry whose lab helped develop the induced pluripotent stem cells (iPSC), added: "It's exciting to be carrying out work that provides hope for HD patients and their families."
Thompson said that UCI scientists will use the new model to study the specific gene expression changes in human brain cells that trigger the onset of HD, helping them understand how these changes happen and how to correct them.
Huntington's disease afflicts about 30,000 people in the U.S. typically striking in midlife and another 75,000 carry the gene that will eventually lead to it. Caused by a mutation in the gene for a protein called huntingtin, the disease damages brain cells so that individuals with HD progressively lose their ability to walk, talk and reason. It invariably culminates in death. While rare, HD is the most common inherited neurodegenerative disease.
###
See the rest here:
Human model of Huntington's disease created from skin's stem cells
Cedars-Sinai Researchers, with Stem Cells and Global Colleagues, Develop Huntington's Research Tool
By daniellenierenberg
Newswise LOS ANGELES (EMBARGOED UNTIL NOON EDT ON JUNE 28, 2012) Cedars-Sinai scientists have joined with expert colleagues around the globe in using stem cells to develop a laboratory model for Huntingtons disease, allowing researchers for the first time to test directly on human cells potential treatments for this fatal, inherited disorder.
As explained in a paper published June 28 on the Cell Stem Cell website and scheduled for print in the journals Aug. 3 issue, scientists at Cedars-Sinais Regenerative Medicine Institute and the University of Wisconsin took skin cells from patients with Huntingtons disease and reprogrammed them into powerful stem cells; these were then made into the nervous system cells affected by the disease. Seven laboratories around the world collaborated to demonstrate the cells had hallmarks of Huntingtons.
This Huntingtons disease in a dish will enable us for the first time to test therapies on human Huntingtons disease neurons, said Clive Svendsen, PhD, director of the Cedars-Sinai Regenerative Medicine Institute and a senior author of the study. In addition to increasing our understanding of this disorder and offering a new pathway to identifying treatments, this study is remarkable because of the extensive interactions between a large group of scientists focused on developing this model. Its a new way of doing trailblazing science.
The Huntingtons Disease iPSC Consortium united some of the worlds top scientists working on this disease. Cedars-Sinai researchers took skin cells from a several Huntingtons patients, including a six-year-old with a severe juvenile form of the disease. They genetically reprogrammed these tissues into induced pluripotent stem cells, which can be made into any type of cell in the body. The cells lines were banked by scientists at Cedars-Sinai and scrutinized by all consortium members for differences that may have led to the disease. These cell lines are now an important resource for Huntingtons researchers and have been made available via a National Institutes of Health-funded repository at Coriell Institute for Medical Research in New Jersey.
Huntingtons, known to the public, for example, as the cause of folksinger Woody Guthries death, typically strikes patients in midlife. It causes jerky, twitching motions, loss of muscle control, psychiatric disorders and dementia; the disease ultimately is fatal. In rare, severe cases, the disorder appears in childhood.
Researchers believe that Huntingtons results from a mutation in the huntintin gene, leading to production of an abnormal protein and ultimately cell death in specific areas of the brain that control movement and cognition. There is no cure for Huntingtons, nor therapies to slow its progression.
The consortium showed Huntingtons cell deficits or how they differ from normal cells, including that they were less likely to survive cultivation in the petri dish. Scientists tried depriving them of a growth factor present around normal cells, or stressing them, and found that Huntingtons neurons died even faster.
It was great that these characteristics were seen not only in our laboratory, but by all of the consortium members using different techniques, said Virginia Mattis, a post-doctoral scientist at the Cedars-Sinai Regenerative Medicine Institute and one of the lead authors of the study. It was very reassuring and significantly strengthens the value of this study.
This new model will provide the foundation for a new round of experiments by the consortium funded by a new grant from the NIH and the California Institute for Regenerative Medicine.
The Cedars-Sinais Regenerative Medicine Institute has made a major commitment to projects like this Huntingtons study in which stem cell research helps to advance understanding of human disease and open new and innovative methods to identify treatments and cures. The institute has developed an induced pluripotent stem cell core facility and recruited faculty to work in this emerging area of regenerative medicine research.
Read more:
Cedars-Sinai Researchers, with Stem Cells and Global Colleagues, Develop Huntington's Research Tool
Turning skin cells into brain cells
By raymumme
Public release date: 28-Jun-2012 [ | E-mail | Share ]
Contact: Stephanie Desmon sdesmon1@jhmi.edu 410-955-8665 Johns Hopkins Medical Institutions
Johns Hopkins researchers, working with an international consortium, say they have generated stem cells from skin cells from a person with a severe, early-onset form of Huntington's disease (HD), and turned them into neurons that degenerate just like those affected by the fatal inherited disorder.
By creating "HD in a dish," the researchers say they have taken a major step forward in efforts to better understand what disables and kills the cells in people with HD, and to test the effects of potential drug therapies on cells that are otherwise locked deep in the brain.
Although the autosomal dominant gene mutation responsible for HD was identified in 1993, there is no cure. No treatments are available even to slow its progression.
The research, published in the journal Cell Stem Cell, is the work of a Huntington's Disease iPSC Consortium, including scientists from the Johns Hopkins University School of Medicine in Baltimore, Cedars-Sinai Medical Center in Los Angeles and the University of California, Irvine, as well as six other groups. The consortium studied several other HD cell lines and control cell lines in order to make sure results were consistent and reproducible in different labs.
The general midlife onset and progressive brain damage of HD are especially cruel, slowly causing jerky, twitch-like movements, lack of muscle control, psychiatric disorders and dementia, and eventually death. In some cases (as in the patient who donated the material for the cells made at Johns Hopkins), the disease can strike earlier, even in childhood.
"Having these cells will allow us to screen for therapeutics in a way we haven't been able to before in Huntington's disease," says Christopher A. Ross, M.D., Ph.D., a professor of psychiatry and behavioral sciences, neurology, pharmacology and neuroscience at the Johns Hopkins University School of Medicine and one of the study's lead researchers. "For the first time, we will be able to study how drugs work on human HD neurons and hopefully take those findings directly to the clinic."
Ross and his team, as well as other collaborators at Johns Hopkins and Emory University, are already testing small molecules for the ability to block HD iPSC degeneration. These small molecules have the potential to be developed into novel drugs for HD.
The ability to generate from stem cells the same neurons found in Huntington's disease may also have implications for similar research in other neurodegenerative diseases such as Alzheimer's and Parkinson's.
Read the rest here:
Turning skin cells into brain cells
Turning skin cells into brain cells: Huntington's disease in a dish
By LizaAVILA
ScienceDaily (June 28, 2012) Johns Hopkins researchers, working with an international consortium, say they have generated stem cells from skin cells from a person with a severe, early-onset form of Huntington's disease (HD), and turned them into neurons that degenerate just like those affected by the fatal inherited disorder.
By creating "HD in a dish," the researchers say they have taken a major step forward in efforts to better understand what disables and kills the cells in people with HD, and to test the effects of potential drug therapies on cells that are otherwise locked deep in the brain.
Although the autosomal dominant gene mutation responsible for HD was identified in 1993, there is no cure. No treatments are available even to slow its progression.
The research, published in the journal Cell Stem Cell, is the work of a Huntington's Disease iPSC Consortium, including scientists from the Johns Hopkins University School of Medicine in Baltimore, Cedars-Sinai Medical Center in Los Angeles and the University of California, Irvine, as well as six other groups. The consortium studied several other HD cell lines and control cell lines in order to make sure results were consistent and reproducible in different labs.
The general midlife onset and progressive brain damage of HD are especially cruel, slowly causing jerky, twitch-like movements, lack of muscle control, psychiatric disorders and dementia, and -- eventually -- death. In some cases (as in the patient who donated the material for the cells made at Johns Hopkins), the disease can strike earlier, even in childhood.
"Having these cells will allow us to screen for therapeutics in a way we haven't been able to before in Huntington's disease," saysChristopher A. Ross, M.D., Ph.D., a professor of psychiatry and behavioral sciences, neurology, pharmacology and neuroscience at the Johns Hopkins University School of Medicine and one of the study's lead researchers. "For the first time, we will be able to study how drugs work on human HD neurons and hopefully take those findings directly to the clinic."
Ross and his team, as well as other collaborators at Johns Hopkins and Emory University, are already testing small molecules for the ability to block HD iPSC degeneration.These small molecules have the potential to be developed into novel drugs for HD.
The ability to generate from stem cells the same neurons found in Huntington's disease may also have implications for similar research in other neurodegenerative diseases such as Alzheimer's and Parkinson's.
To conduct their experiment, Ross took a skin biopsy from a patient with very early onset HD.When seen by Ross at the HD Center at Hopkins, the patient was just seven years old. She had a very severe form of the disease, which rarely appears in childhood, and of the mutation that causes it. Using cells from a patient with a more rapidly progressing form of the disease gave Ross' team the best tools with which to replicate HD in a way that is applicable to patients with all forms of HD.
Her skin cells were grown in culture and then reprogrammed by the lab of Hongjun Song, Ph.D., a professor at Johns Hopkins' Institute for Cell Engineering, into induced pluripotent stem cells. A second cell line was generated in an identical fashion in Dr. Ross's lab from someone without HD. Simultaneously, other HD and control iPS cell lines were generated as part of the NINDS funded HD iPS cell consortium.
Read the original here:
Turning skin cells into brain cells: Huntington's disease in a dish
Disease fight: turning skin cells to neurons
By LizaAVILA
Disease fight: turning skin cells to neurons June 28th, 2012, 4:04 pm posted by Pat Brennan, science, environment editor
UC Irvine professor Leslie Thompson, with human brain image behind her. Photo by Daniel A. Anderson, UC Irvine.
Using stem cells derived from skin cells, scientists including a UC Irvine team say they have created human neurons that exhibit the effects of Huntingtons disease promising the possibility of testing treatments for the deadly disorder in a petri dish.
Their discovery not only sidesteps ethical issues surrounding the use of human embryonic stem cells, but offers the chance to produce far more diseased neurons, at various stages of disease progression, than ever have been available to researchers before.
This is a relatively new technique where you can reprogram an adult cell, in this case a skin cell, back to this early stem-cell stage, and then guide those into making neurons, said Leslie Thompson, a UC Irvine professor and a senior author of a study announcing the discovery that was published online Thursday.
Huntingtons disease is an inherited, neurodegenerative disorder that is always fatal. It typically strikes in middle age, gradually robbing its victims of the ability to walk and interfering with other basic brain functions.
Huntington's disease cells on their way to becoming neurons. Image courtesy Leslie Thompson, UC Irvine.
Its like Parkinsons in that its a movement disorder in this case, involuntary movements, and rigidity, Thompson said. You know what is going on, but parts of memory are being impaired; you have an impaired ability to walk, think, talk.
Victims typically die of the diseases effects falling, or choking during pneumonia and some especially severe mutations can strike young children. The disease affects about 30,000 people in the United States, and no treatments exist even to slow the onset of symptoms.
The scientists, including UCIs Leslie Lock and Peter Donovan, director of the Sue and Bill Gross Stem Cell Research Center, as well as others from universities around the country and in Italy and Great Britain, used a variety of cell lines to reveal the genetic underpinnings of Huntingtons.
Read the original here:
Disease fight: turning skin cells to neurons
Speeding Up Bone Growth by Manipulating Stem Cells
By daniellenierenberg
Newswise If you break a bone, you know you'll end up in a cast for weeks. But what if the time it took to heal a break could be cut in half? Or cut to just a tenth of the time it takes now? Qian Wang, a chemistry professor at the University of South Carolina, has made tantalizing progress toward that goal.
Wang, Andrew Lee and co-workers just reported in Molecular Pharmaceutics that surfaces coated with bionanoparticles could greatly accelerate the early phases of bone growth. Their coatings, based in part on genetically modified Tobacco mosaic virus, reduced the amount of time it took to convert stem cells into bone nodules from two weeks to just two days.
The key to hastening bone healing or growth is to coax a perfectly natural process to pick up the pace.
"If you break a rib, or a finger, the healing is automatic," said Wang. "You need to get the bones aligned to be sure it works as well as possible, but then nature takes over."
Healing is indeed very natural. The human body continuously generates and circulates cells that are undifferentiated; that is, they can be converted into the components of a range of tissues, such as skin or muscle or bone, depending on what the body needs.
The conversion of these cells called stem cells is set into motion by external cues. In bone healing, the body senses the break at the cellular level and begins converting stem cells into new bone cells at the location of the break, bonding the fracture back into a single unit. The process is very slow, which is helpful in allowing a fracture to be properly set, but after that point the wait is at least an inconvenience, and in some cases highly detrimental.
"With a broken femur, a leg, you can be really incapacitated for a long time," said Wang. "In cases like that, they sometimes inject a protein-based drug, BMP-2, which is very effective in speeding up the healing process. Unfortunately, it's very expensive and can also have some side effects."
In a search for alternatives four years ago, Wang and colleagues uncovered some unexpected accelerants of bone growth: plant viruses. They originally meant for these viruses, which are harmless to humans, to work as controls. They coated glass surfaces with uniform coverings of the Turnip yellow mosaic virus and Tobacco mosaic virus, originally intending to use them as starting points for examining other potential variations.
But they were surprised to find that the coatings alone could reduce the amount of time to grow bone nodules from stem cells. Since then, Wang and co-workers have refined their approach to better define just what it is that accelerates bone growth.
Over the course of the past four years, they've demonstrated that it's a combination of the chemistry as well as the topography of the surface that determines how long it takes a stem cell to form bone nodules. The stem cells are nestled into a nanotopgraphy defined by the plant virus, and within that nanotopography the cells make contact with the variety of chemical groups on the viral surface.
See original here:
Speeding Up Bone Growth by Manipulating Stem Cells
Beating Cardiomyocytes: From skin cells to stem cells
By raymumme
22-06-2012 13:13 This is a small group of beating heart cells in a cell culture that was derived from non-embryonic pluripotent stem cells ("induced pluripotent stem cells"). The induced pluripotent stem cells were generated from skin fibroblasts that were isolated from an 87 year old Native American female. This movie was made through a microscope- the entire culture is only about a millimeter (4 hundredths of an inch) across. The cultures were produced by members of the Loring Lab at The Scripps Research Institute in La Jolla, California.
See more here:
Beating Cardiomyocytes: From skin cells to stem cells
Stem Cells To Aid In Heart-Related Research
By NEVAGiles23
June 21, 2012
Connie K. Ho for redOrbit.com
Pumping vigorously night and day, the heart is clearly one of the most important organs in the human body. It is also one of the most delicate parts of the body. As such, news regarding heart-related diseases is beneficial to both doctors and patients. University of Michigan (UM) researchers recently reported the discovery of a new method that could produce cardiac muscle patches from stem cells.
The innovative process was created at UMs Center for Arrhythmia Research and effectively uses stem cells that can copy the hearts squeezing action. The cells showed activity that was like that of peoples resting heart rate. The rhythmic electrical impulse transmission of the engineered cells worked at a rate of 60 beats per minute and this rate was 10 times quicker than rates reported in other stem cell studies.
To date, the majority of studies using induced pluripotent stem cell-derived cardiac muscle cells have focused on single cell functional analysis, remarked senior author Dr. Todd J. Herron, an assistant research professor in the Departments of Internal Medicine and Molecular & Integrative Physiology at the U-M, in a prepared statement.
The researchers believe that the stem biology findings will be beneficial to those who suffer from common but life-threatening heart diseases. They hope that the use of stem cells will assist patients diagnosed with arrhythmia, which is found in approximately 2.5 million people. With arrhythmia, patients suffer an irregularity in the hearts electrical impulses and this can hinder the hearts ability to circulate blood.
For potential stem cell-based cardiac regeneration therapies for heart disease, however, it is critical to develop multi-cellular tissue like constructs that beat as a single unit, commented Herron in the statement.
Regarding the specifics of the project, the goal of the scientists was to use stem cells to develop skin biopsies. These biopsies could be used to produce large quantities of cardiac muscle cells, which could then help transmit uniform electrical impulses and work as a cohesive unit. In collaborating with researchers from the University of Oxford, Imperial College, and the University of Wisconsin, the team was able to design a fluorescent imaging platform. The platform used light emitting diode (LED) illumination to quantify the cells electrical activity.
Action potential and calcium wave impulse propagation trigger each normal heart beat, so it is imperative to record each parameter in bioengineered human cardiac patches, remarked Herron in the statement.
Overall, authors of the study believe that the velocity of the engineered cardiac cells is still slower than the velocity of cells found in the beating adult heart. However, the velocity of the engineered cardiac cells is quicker than those previously reported; it is also similar to the rate found in commonly used rodent cells. For future scientific research purposes, the investigators theorize that human cardiac patches could be utilized instead of rodent systems. The new method could be used in many cardiac research laboratories and allow cardiac stem cell patches to be utilized in disease research, new drug treatment testing, and therapies focused on repairing damaged heart muscles.
Read more:
Stem Cells To Aid In Heart-Related Research
Scientists grow tiny liver in mouse's head
By raymumme
Using stem cells from human skin, Japanese scientists have grown a small human liver inside the skull of a mouse.
Hideki Taniguchi and Takanori Takebe from Yokohama City University used stem cells generated from human skin cells and developed them into percussor liver cells, the New Scientist reports.
Then they added other cells from umbilical cord blood vessels. The combination of cells then "guided itself" to form a small structure similar to liver tissue, Takebe said.
"We mixed and graded the cells onto the culture dish and they moved to form a cluster," he said. "It was a surprising outcome from what was, to be honest, an accident."
They implanted the structure into the head of a mouse, which was suffering from a severe genetic immune system disorder that prevented it from having an immune reaction to the foreign tissues.
The increased blood flow in the mouse's skull allowed the tissue to keep growing.
Within 48 hours, human blood vessels and human proteins formed. Glycogen and amino acids levels were the same as those of a human liver.
"It's not yet a perfect liver," Takebe said. "Improvements need to be made, such as the reconstruction of a bile duct."
The study could be significant for the field of regenerative medicine, but the researchers aren't yet sure whether the organ is a fully functioning liver, or whether they will be able to scale it to human size.
The findings were presented at the at the International Society for Stem Cell Research's annual meeting in Yokohama.
Read the original:
Scientists grow tiny liver in mouse's head
Makucell Unveils Renewnt™, a Revolutionary, Science-driven Skin Care Brand
By NEVAGiles23
SCOTTSDALE, Ariz. & LOS ANGELES--(BUSINESS WIRE)--Makucell, Inc., a pioneering regenerative biotechnology company dedicated to the development, manufacture and distribution of non-prescription products formulated to address the impact of aging and photo-damaged skin unveils the Renewnt (pronounced Re-new-int) brand, a revolutionary science-driven product line. Renewnts proprietary ingredient, Asymmtate, is a new approach to cellular aging, optimizing signals in the Wnt (pronounced wint) pathway to energize the skins stem cells, encouraging youthful cell behavior. The result is younger-looking skin which appears firmer and smoother. This molecular process is the key to our proprietary technology developed at USCs Keck School of Medicine and transferred to Makucells Renewnt skin care line.
We conducted standard industry safety tests, and the results were universally positive; Renewnt products were well tolerated with no adverse effects or safety issues. A combination of clinical trials and in vitro gene expression studies from treated biopsied skin continues to corroborate the aesthetic effects noted in the clinic.
The Makucell Science
The bodys signals govern skin stem cells, controlling the decision to remain dormant, divide or differentiate (become normal, active tissue cells). Signals flow in pathways and multiple paths converge into one the Wnt pathway. Makucells proprietary molecule Asymmtate encourages optimal signaling in the Wnt pathway. Optimal signaling stimulates the skin stem cells to begin the process leading to keratinocytes, fibroblasts and other dermal cells which produce collagen, elastic tissue and substances in the supporting skin matrix. This essential regenerative process is the key differentiator in Makucells Renewnt skin care products.
Michael Kahn, Ph.D. and his team of gifted research scientists at the Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research of the Keck School of Medicine at the University of Southern California developed the revolutionary ingredient, Asymmtate, Makucells core technology. Dr. Mark V. Dahl, Makucell Chief Medical Officer and former President of the American Academy of Dermatology as well as Professor Emeritus at the Mayo Clinic in Arizona, developed the formulations designed to target a specific skin type on a particular area of the body. Makucell has tested all the Renewnt products for safety and efficacy. The products:
Makucell is committed to supporting our claims with results from controlled, blinded studies, explains Dr. Dahl. We conducted standard industry safety tests, and the results were universally positive; Renewnt products were well tolerated with no adverse effects or safety issues. A combination of clinical trials and in vitro gene expression studies from treated biopsied skin continues to corroborate the aesthetic effects noted in the clinic.
Dr. Lawrence Rheins, President and Chief Executive Officer of Makucell, commented, Renewnt delivers extraordinary regenerative ability in a hydrating cream, providing an advanced anti-aging option. Asymmtate in Renewnt wakes-up the skins stem cells which have become sluggish with age, to begin rebuilding the underlying supporting skin matrix. As a result, skin looks plumper and has a rejuvenated, youthful appearance.
Makucells current Renewnt skin care product line includes:
Renewnt for Hydration, a day and night facial moisturizer for a more youthful-looking appearance.
Renewnt for Strength, for the dry, thinning skin on hands and forearms to seal in moisture, repair the signs of aging and restore the essential skin barrier.
Read more:
Makucell Unveils Renewnt™, a Revolutionary, Science-driven Skin Care Brand


 September 26th, 2012
September 26th, 2012