Actinium Highlights Iomab-B Safety Data Presented at the 62nd American Society of Hematology Annual Meeting – PRNewswire
By daniellenierenberg
NEW YORK, Dec. 7, 2020 /PRNewswire/ --Actinium Pharmaceuticals, Inc. (NYSE AMERICAN: ATNM) ("Actinium" or the "Company") today announced that safety data from its ongoing pivotal Phase 3 SIERRA trial of Iomab-B in patients with relapsed or refractory Acute Myeloid Leukemia (R/R AML) were presented at the 2020 American Society of Hematology (ASH) annual meeting. The oral presentation highlighted Iomab-B's targeting ability and corresponding safety data from 110 patients from the SIERRA trial for which detailed safety data was available. Iomab-B targets CD45, an antigen expressed on leukemia and lymphoma cancer cells and immune cells including bone marrow stem cells but not cells outside of the blood forming or hematopoietic system. This allows high amounts of radiation to be delivered to the bone marrow via Iomab-B while sparing healthy organs. As a result, statistically significant lower rates of sepsis were reported as well as lower rates of febrile neutropenia, mucositis and non-relapse transplant related mortality in patients receiving Iomab-B and bone marrow transplant (BMT) compared to patients that received salvage therapy and a BMT. In addition, patients that crossed over to receive Iomab-B and went to BMT after receiving salvage therapy but not achieving a complete response also had lower rates of sepsis, febrile neutropenia, mucositis and non-relapse transplant related mortality.
Dr. Mark Berger, Actinium's Chief Medical Officer, commented, "We are pleased that the engraftment and safety profile of Iomab-B remains positive and consistent with prior interim safety results at 75% of patient enrollment in SIERRA and also consistent with the large body of historical data from Iomab-B. Collectively, this data gives excitement as we approach the upcoming ad hoc interim analysis for SIERRA that will be completed by year-end and the ultimate potential of Iomab-B for patients with R/R AML and other blood cancers as a targeted conditioning regimen."
Safety data presented in ASH oral presentation are highlighted in the table below:
ASH Oral Presentation:High Doses of Targeted Radiation with Anti-CD45 Iodine (131I) Apamistamab [Iomab-B] Do Not Correlate with Incidence of Mucositis, Febrile Neutropenia or Sepsis in the Prospective, Randomized Phase 3 Sierra Trial for Patients with Relapsed or Refractory Acute Myeloid Leukemia
Adverse Event
Received Iomab-B/HCT (N=47)1% (N)
No CR Crossed over to Iomab-B/HCT (N=30)2% (N)
Achieved CR and received Std HCT (N=9) % (N)
Sepsis
4.3 (2)
22.2 (6)
33.3 (3)
Febrile Neutropenia Gr 3-4
34.8 (16)
40.7 (11)
55.6 (5)
Mucositis Gr 3-4
10.9 (5)
18.5 (5)
33.3 (3)
Day +100 Non-Relapse Mortality3
2/45
(4.4%)
3/26
(11.5%)
2/9
(22.2%)
1 Adverse Event data available for 46 of 47 evaluable patients
2 Adverse Event data available for 27 of 30 evaluable patients
3 Iomab-B arm: 4 patients unevaluable. Conventional Care Arm: 4 patients unevaluable
Patient Group
No. of Patients
Radiation dose delivered to the Marrow. Median (range)
Radiation dose to GI tract. Median (range)
Iomab-B
47
14.9 Gy
(4.6-32)
2.8 Gy
(1.6-6.7)
Vijay Reddy, Vice President, Clinical Development and Head of BMT, "The targeted nature of Iomab-B makes it highly differentiated from current BMT conditioning regimens that are largely comprised of non-targeted cytotoxic chemotherapies. These data from SIERRA showing higher rates of sepsis, neutropenia and mucositis in patients receiving chemotherapy are consistent with the literature and unfortunately what we expected but hope to address with Iomab-B. Particularly, chemotherapy's effect on the GI tract and resulting mucositis, which we believe is leading to the higher rates of sepsis seen in the control arm. We are highly encouraged by the lower rates of adverse events and the universal engraftment reported from SIERRA and excited for the potential of targeted conditioning could have an BMT access, patient outcomes and quality of life."
About Iomab-B
Iomab-B (I-131 apamistamab) via the monoclonal antibody apamistamab, targets CD45, an antigen widely expressed on leukemia and lymphoma cancer cells, B cells and stem cells. Apamistamab is linked to the radioisotope iodine-131 (I-131) and once attached to its target cells emits energy that travels about 100 cell lengths, destroying a patient's cancer cells and ablating their bone marrow. By carrying iodine-131 directly to the bone marrow in a targeted manner, Actinium believes Iomab-B will avoid the side effects of radiation on most healthy tissues while effectively killing the patient's cancer and marrow cells.
Iomab-B is currently being studied in the pivotal Phase 3 SIERRA (Study of Iomab-B in Relapsed or Refractory AML) trial, a 150-patient, randomized controlled clinical trial in patients with relapsed or refractory Acute Myeloid Leukemia (AML) who are age 55 and above. The SIERRA trial is being conducted at preeminent transplant centers in the U.S. with the primary endpoint of durable Complete Remission (dCR) at six months and a secondary endpoint of overall survival at one year. Upon approval, Iomab-B is intended to prepare and condition patients for a bone marrow transplant, also referred to as a hematopoietic stem cell transplant, in a potentially safer and more efficacious manner than the non-targeted intensive chemotherapy conditioning that is the current standard of care in bone marrow transplant conditioning. A bone marrow transplant is often considered the only potential cure for patients with certain blood-borne cancers and blood disorders. Additional information on the Company's Phase 3 clinical trial in R/R can be found at http://www.sierratrial.com.
About Actinium Pharmaceuticals, Inc. (NYSE: ATNM)
Actinium Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company developing ARCs or Antibody Radiation-Conjugates, which combine the targeting ability of antibodies with the cell killing ability of radiation. Actinium's lead application for our ARCs is targeted conditioning, which is intended to selectively deplete a patient's disease or cancer cells and certain immune cells prior to a BMT or Bone Marrow Transplant, Gene Therapy or Adoptive Cell Therapy (ACT) such as CAR-T to enable engraftment of these transplanted cells with minimal toxicities. With our ARC approach, we seek to improve patient outcomes and access to these potentially curative treatments by eliminating or reducing the non-targeted chemotherapy that is used for conditioning in standard practice currently. Our lead product candidate, I-131 apamistamab (Iomab-B) is being studied in the ongoing pivotal Phase 3 Study of Iomab-B in Elderly Relapsed or Refractory Acute Myeloid Leukemia (SIERRA) trial for BMT conditioning. The SIERRA trial is over seventy-five percent enrolled and positive single-agent, feasibility and safety data has been highlighted at ASH, TCT, ASCO and SOHO annual meetings. More information on this Phase 3 clinical trial can be found at http://www.sierratrial.com. I-131 apamistamab will also be studied as a targeted conditioning agent in a Phase 1 study with a CD19 CAR T-cell therapy and in a Phase 1/2 anti-HIV stem cell gene therapy with UC Davis. In addition, we are developing a multi-disease, multi-target pipeline of clinical-stage ARCs targeting the antigens CD45 and CD33 for targeted conditioning and as a therapeutic either in combination with other therapeutic modalities or as a single agent for patients with a broad range of hematologic malignancies including acute myeloid leukemia, myelodysplastic syndrome and multiple myeloma. Ongoing combination trials include our CD33 alpha ARC, Actimab-A, in combination with the salvage chemotherapy CLAG-M and the Bcl-2 targeted therapy venetoclax. Underpinning our clinical programs is our proprietary AWE (Antibody Warhead Enabling) technology platform. This is where our intellectual property portfolio of over 130 patents, know-how, collective research and expertise in the field are being leveraged to construct and study novel ARCs and ARC combinations to bolster our pipeline for strategic purposes. Our AWE technology platform is currently being utilized in a collaborative research partnership with Astellas Pharma, Inc. Website: http://www.actiniumpharma.com
Forward-Looking Statements for Actinium Pharmaceuticals, Inc.
This press release may contain projections or other "forward-looking statements" within the meaning of the "safe-harbor" provisions of the private securities litigation reform act of 1995 regarding future events or the future financial performance of the Company which the Company undertakes no obligation to update. These statements are based on management's current expectations and are subject to risks and uncertainties that may cause actual results to differ materially from the anticipated or estimated future results, including the risks and uncertainties associated with preliminary study results varying from final results, estimates of potential markets for drugs under development, clinical trials, actions by the FDA and other governmental agencies, regulatory clearances, responses to regulatory matters, the market demand for and acceptance of Actinium's products and services, performance of clinical research organizations and other risks detailed from time to time in Actinium's filings with the Securities and Exchange Commission (the "SEC"), including without limitation its most recent annual report on form 10-K, subsequent quarterly reports on Forms 10-Q and Forms 8-K, each as amended and supplemented from time to time.
Contacts:
Investors:Clayton Robertson Actinium Pharmaceuticals, Inc. [emailprotected]
Hans Vitzthum LifeSci Advisors, LLC[emailprotected](617) 430-7578
SOURCE Actinium Pharmaceuticals, Inc.
http://www.actiniumpharma.com/
See the article here:
Actinium Highlights Iomab-B Safety Data Presented at the 62nd American Society of Hematology Annual Meeting - PRNewswire
ElevateBio’s HighPassBio Presents on Novel T Cell Receptor Cell Therapy for Leukemia Relapse at 62nd Annual ASH Meeting – Business Wire
By daniellenierenberg
CAMBRIDGE, Mass.--(BUSINESS WIRE)--HighPassBio, an ElevateBio portfolio company dedicated to advancing novel targeted T cell immunotherapies, today discussed the ongoing Phase 1 trial of the companys lead product candidate, an engineered T cell receptor (TCR) T cell therapy targeting HA-1 expressing cancer cells in an oral presentation at the 62nd American Society of Hematology (ASH) Annual Meeting. The Phase 1 clinical trial, which is being conducted by researchers at Fred Hutchinson Cancer Research Center, is designed to assess the feasibility, safety, and efficacy of this novel cell therapy in the treatment of leukemia following hematopoietic stem cell transplant (HSCT).
The prognosis for leukemia patients whove relapsed or who have residual disease following allogeneic hematopoietic stem cell transplantation is often poor, but we believe that by targeting the minor H antigen, HA-1, through a novel T cell immunotherapy, we can potentially treat and prevent subsequent relapse, said Elizabeth Krakow, M.D., MSc., Assistant Professor, Clinical Research Division, Fred Hutchinson Cancer Research Center, principal investigator of the study, and presenting author. We have observed early promising indicators of anti-leukemic activity following treatment in this trial. We are eager to expand the trial to additional patients as we continue to research the feasibility, safety, and efficacy of this approach.
The abstract for the presentation titled Phase 1 Study of Adoptive Immunotherapy with HA-1-Specific CD8+ and CD4+ Memory T Cells for Children and Adults with Relapsed Acute Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation (HCT): Trial in Progress, can be found on the ASH website under the abstract number 137726.
To date, four patients, including one pediatric patient, have received a total of six infusions in the Phase 1 clinical trial. Patient characteristic data was shared in the oral presentation at ASH, including documented HA-1 TCR T cell persistence in blood and bone marrow up to 18 months. In some patients, clear in vivo anti-leukemic activity was observed at the first dose level, including a subject with aggressive, highly refractory T-ALL and early post-HCT relapse. No significant toxicities attributed to the T cells have been observed, including no infusion reactions or evidence of cytokine release syndrome or graft versus host disease.
The Phase 1 clinical trial is currently recruiting adult and pediatric patients who have residual disease or relapsed leukemia or related conditions following HSCT. As part of the trial, transplant patients and prospective donors may be recruited to participate in the genetic screening portion to determine eligibility. More details are available on clinicaltrials.gov under the study ID number NCT03326921.
About TCR-Engineered T Cell Therapy
A key role of the immune system is to detect tumor antigens, engage T cells, and eradicate the tumor. However, the immune response to tumor antigens varies and is often insufficient to prevent tumor growth and relapse. An approach known as adoptive T cell therapy, using T cell receptors, or TCRs, can overcome some of the obstacles to establishing an effective immune response to fight off the target tumor. TCRs are molecules found on surface of T cells that can recognize tumor antigens that are degraded to small protein fragments inside tumor cells. Unlike CAR T cells that recognize only surface antigens, TCRs can recognize small protein fragments derived from intracellular and surface antigens offering a more diverse way to attack tumors. These small protein fragments show up on the tumor cell surface, with another protein called major histocompatibility complex (MHC), that are recognized by the TCRs and consequently signal the bodys immune system to respond to fight off and kill the tumor cells.
Tumor-specific TCRs can be identified and then engineered into T cells that recognize and attack various types of cancers, representing a novel approach to treating and potentially preventing disease.
Adoptive T cell therapy can be applied to tackling relapse of leukemia post hematopoietic stem cell transplant (HSCT) by targeting the antigens expressed only by the patients native cells, and not by the cells from the stem cell transplant donor. HA-1, a known minor histocompatibility antigen, is expressed predominantly or exclusively on hematopoietic cells, including leukemic cells. There is evidence that T cells specific for HA-1 can induce a potent and selective antileukemic effect. HA-1 TCR T cell therapy is a new investigational immunotherapy for the management of post transplantation leukemia relapse.
About Leukemia post HSCT Treatment and the Risk of Relapse
Leukemia, a cancer of the blood or bone marrow characterized by an abnormal proliferation of blood cells, is the tenth most common type of cancer in the U.S. with an estimated 60,140 new cases and 24,400 deaths in 2016. Leukemia arises from uncontrolled proliferation of a specific type of hematopoietic (blood) cell that is critical for a functional immune system. As a result, when patients are given very high doses of chemotherapy to eradicate leukemic cells, most normal cells are killed as well, necessitating a transplant of hematopoietic stem cells from a donor to reconstitute the patients bone marrow and circulating hematopoietic cells. In some cases, the transplanted T cells from the donor can also recognize and eliminate the hematopoietic cells, including leukemia, from the recipient, thus preventing relapse. This can be described as a graft versus leukemia effect. Other hematologic disorders related to leukemia, like myelodysplastic syndrome (MDS), can also be treated in this way.
While HSCT can be curative, it is estimated that 25-50 percent of HSCT recipients relapse; leukemia relapse remains the major cause of allogeneic HSCT failure, and the prognosis for patients with post-HCT relapse is poor. Relapse occurs following allogeneic HSCT in approximately one-third of patients with acute leukemia who undergo the procedure, and most patients subsequently die of their disease.
About HighPassBio
HighPassBio, an ElevateBio portfolio company, is working to advance a novel approach to treating hematological malignancies by leveraging T cell receptor (TCR)-engineered T cells, known as TCR T cells. The companys lead program is designed to treat or potentially prevent relapse of leukemia in patients who have undergone hematopoietic stem cell transplant (HSCT). The technology was born out of research conducted at Fred Hutchinson Cancer Research Center by world renowned expert, Dr. Marie Bleakley.
About ElevateBio
ElevateBio, LLC, is a Cambridge-based creator and operator of a portfolio of innovative cell and gene therapy companies. It begins with an environment where scientific inventors can transform their visions for cell and gene therapies into reality for patients with devastating and life-threatening diseases. Working with leading academic researchers, medical centers, and corporate partners, ElevateBios team of scientists, drug developers, and company builders are creating a portfolio of therapeutics companies that are changing the face of cell and gene therapy and regenerative medicine. Core to ElevateBios vision is BaseCamp, a centralized state-of-the-art innovation and manufacturing center, providing fully integrated capabilities, including basic and translational research, process development, clinical development, cGMP manufacturing, and regulatory affairs across multiple cell and gene therapy and regenerative medicine technology platforms. ElevateBio portfolio companies, as well as select strategic partners, are supported by ElevateBio BaseCamp in the advancement of novel cell and gene therapies.
ElevateBios investors include F2 Ventures, MPM Capital, EcoR1 Capital, Redmile Group, Samsara BioCapital, The Invus Group, Surveyor Capital (A Citadel company), EDBI, and Vertex Ventures.
ElevateBio is headquartered in Cambridge, Mass, with ElevateBio BaseCamp located in Waltham, Mass. For more information, please visit http://www.elevate.bio.
Read the rest here:
ElevateBio's HighPassBio Presents on Novel T Cell Receptor Cell Therapy for Leukemia Relapse at 62nd Annual ASH Meeting - Business Wire
ASH virtual event hears about CRISPR and CAR-T based approaches to hard-to-treat blood disorders and cancers – BioPharma-Reporter.com
By daniellenierenberg
In the first study, researchers used CRISPR/Cas9 to treat two inherited blood disorders, beta thalassemia and sickle cell disease (SCD). The trial, which demonstrated remarkable improvements in all participants, is the first time this revolutionary approach has been used successfully in these patient populations.
Given that the only FDA-approved cure for sickle cell disease, a bone marrow transplant, is not widely accessible, having another curative option would be life-changing for a large number of the sickle cell disease population, said press briefing moderator, Dr Catherine Bollard, of Childrens National Research Institute and George Washington University. While longer follow-up data are needed, this study is extremely exciting for the field.
Investigators reported interim safety and efficacy data from 10 patients who received an investigational gene-editing based therapy, CTX001. The trials are the first to test a CRISPR-Cas9 gene editing therapy in humans for a genetic disease, the researchers reported.
Sickle cell disease (SCD) can cause a variety of health problems including episodes of severe pain, called vaso-occlusive crises, as well as organ damage and strokes, while patients with transfusion-dependent thalassemia (TDT) are dependent on blood transfusions from early childhood.The only available cure for both diseases is a bone marrow transplant from a closely related donor, an option that is not available for the vast majority of patients because of difficulty locating matched donors, the cost, and the risk of complications.
In the studies, the researchers goal is to functionally cure the blood disorders using CRISPR/Cas9 gene-editing by increasing the production of fetal hemoglobin, which produces normal, healthy red blood cells as opposed to the misshapen cells produced by faulty hemoglobin in the bodies of individuals with the disorders.
The clinical trials involve collecting stem cells from the patients. Researchers edit the stem cells using CRISPR-Cas9 and infuse the gene-modified cells into the patients. Patients remain in the hospital for approximately one month following the infusion.
Prior to receiving their modified cells, the seven patients with beta thalassemia required blood transfusions around every three to four weeks and the three patients with SCD suffered episodes of severe pain roughly every other month.
All the individuals with beta thalassemia have been transfusion independent since receiving the treatment, a period ranging between two and 18 months. Similarly, none of the individuals with SCD have experienced vaso-occlusive crises since CTX001 infusion.
All patients showed a substantial and sustained increase in the production of fetal hemoglobin.
Researchers report that the safety of CTX001 infusion was generally consistent with the chemotherapy regimen received prior to cell infusion.
Four serious adverse events (SAEs) related or possibly related to CTX001 were reported in one patient with TDT: headache, haemophagocytic lymphohistiocytosis (HLH), acute respiratory distress syndrome, and idiopathic pneumonia syndrome. All four of these SAEs occurred in the context of HLH and were either resolved or clinically improving at the time of this analysis. No other CTX001-related SAEs were reported in the other patients with TDT or in any patients with SCD, said the investigators.
Haydar Frangoul, MD, Medical Director of Pediatric Hematology and Oncology at Sarah Cannon Research Institute, HCA Healthcares TriStar Centennial Medical Center, said:What we have been able to do through this study is a tremendous achievement. By gene editing the patients own stem cells we may have the potential to make this therapy an option for many patients facing these blood diseases.
Because of the precise way CRISPR-Cas9 gene editing works, Dr Frangoul suggested the technique could potentially cure or ameliorate a variety of diseases that have genetic origins.
The trial was sponsored by CRISPR Therapeutics and Vertex Pharmaceuticals.
The second two studies indicate new opportunities to reach a broader patient population with chimeric antigen receptor T-cell (CAR-T) therapy, which has been shown to be effective in some blood cancers but does not work in all patients.
One of the new studies offers an explanation as to why some patients do not respond to CD19-CAR-T therapy and suggests a way to overcome this resistance. The other study suggests CD19-CAR-T may be a viable option for some patients with high-risk non-Hodgkin lymphoma who have not responded to standard treatments.
Getting more data on CD19-CAR-T therapy in the high-risk non-Hodgkin lymphoma population is very important, said Dr Bollard. We know that CD19-CAR-T therapy does not work for some patients, so these studies underscore the need to better understand the immune evasion mechanisms T cells might be susceptible to and not just focus on their role as a vehicle for the CAR. Doing so may improve our capacity to administer effective T-cell immunotherapies.
Read the original:
ASH virtual event hears about CRISPR and CAR-T based approaches to hard-to-treat blood disorders and cancers - BioPharma-Reporter.com
Negrin Shines Light on the Orca-T Story in GVHD – OncLive
By daniellenierenberg
What started out as a journey to better understand regulatory T cells has now led to an intriguing approach with an investigational cell therapy designed to prevent the risk of graft-versus-host disease (GVHD) and to improve relapse-free survival rates in patients undergoing hematopoietic stem cell transplantation (HSCT).
Data of a phase 1/2 trial recently showed that the first-generation precision cell treatment Orca-T compared with a historical control of standard HSCT demonstrated faster neutrophil (median, 12 days vs 14 days; P < .0001) and platelet engraftment (median, 11 days vs 17 days; P < .0001), decreased incidence of grade 2 or higher GVHD at 100 days (10% vs 30%, P = .005) and chronic GVHD at 1 year (3% vs 46%, P = .0002).1,2
The 1-year GVHD-free and GVHD relapse-free survival (GRFS) rates were 75% with the use of Orca-T vs 31% with standard HSCT (P < .0001). The comparator cohort was derived from contemporaneous patients who had been treated at Stanford University with a conventional allograft.
Along with feasibility of the approach, the results also highlight how Orca-T demonstrates potent anti-leukemic activity in patients who have active disease at HSCT, which suggests that the decrease of GVHD does not impact graft-vs-leukemia (GvL).
That is the most exciting part about the Orca-T story; it is the ability to do this with precision, with speed, and to export it to other sites. The results are intriguing, and very supportive, said Robert Negrin, a professor of medicine (blood and marrow transplantation), and chief of the Division of Blood and Marrow Transplantation at Stanford University.
In an interview with OncLive, Negrin, who is senior author on the trial, shared the evolution of Orca-T as a novel approach to HSCT, highlighted his robust experience with using this cell therapy at Stanford University, and how Orca-T is a potential prevention method for GVHD.
OncLive: Please provide some background to this therapeutic approach. What is the mechanism of action? How is it effective in patients undergoing transplant?
Negrin: This whole idea came from mouse studies many, many years ago, where we identified GVHD as being a dysregulated immune reaction that just keeps going, and going, and going. Like you and I, when we react to something, we have a reactionlet's say, influenza. Our body responds, and then we stop reacting and you get better. With GVHD, what we noticed in using a bioluminescent animal model is that the alloreactive T cells just keep going, going, and going and are unrelenting in mice, just like in people. The problem is very similar and affects certain organs in a very similar way.
Therefore, we went about trying to understand the use of so-called regulatory cells. These are cells that everybody has that help control immune reactions. We just applied them in this clinical scenario, first in mice work done by Matthias Edinger, MD, when he was a postdoctoral fellow many years ago [and other researchers]. All of them were very actively involved in these studies, and showed, somewhat surprisingly, that the administration of regulatory T cells could control this dysregulated immune response that we called GVHD.
Probably more surprising was that, at least in the animal models, it also allowed for the benefits of transplant, namely, the graft-vs-tumor effect and better immune recovery. This was in large part because GVHD also impacts the immune repertoire and where the immunity is developed in the recipient.
All of this was very nice in mouse models and was very elegant. We did a lot of studies, published a number of nice papers, and thought this would be a great idea because it sort of solved, or at least addressed, the principal problems after bone marrow transplantationnamely, avoidance of GVHD yet retention of graft-versus-tumor effects and better immunity. A lot of times, people say, "Oh, that sounds good in mice, but, that's too good to be true." And, theyll ask, "Will that all work in people?"
Where did the biggest challenges lie in this approach?
The big challenge came about to try to apply this to patients. We also have one other interesting point that is relevant. If we gave the regulatory T cells first, before the so-called conventional CD4+/CD8+ cells, that allowed for a lower dose of regulatory T cells. This is because a big challenge is the paucity of these cells; you and I don't have that many.
Then, the other big challenge was the technical ability to isolate in cells. What we do in mice is cell sorting, which is a standard technology. But, that was not developed in people because we're bigthere are a lot of cells, and cell sorting is rather slow, and it's very specific. To get enough cells takes a really long time. It's somewhat of a heroic thing to do in people, to get the adequate amount ourselves; of course, we don't really know what this proper cell dose is.
However, what we thought we learned was that the ratio of conventional to regulatory T cells was the key component. Also, if you give the regulatory T cells first, you can get fewer numbers. Those are things you can do in transplant. You can get the cell from the donor, and you can give cells in a certain sequence; all of those things are very doable. It seemed like an attractive thing to do in patients.
Then, the question was: Does it work? There are 3 groups that have really pioneered this work. The first study came from the University of Perugia in Italy. They did this in haploidentical transplantation; you cannot avoid immunosuppression in haploidentical transplants. They were able to show in several nice papers that you could do this strategy, and seemingly, get away with low risk of GVHD, and also low relapse. This is because the other issue is: how do you measure the graft-vs-tumor effect? There is no assay, and we have no test; you have to wait and see who relapses and who doesn't. Therefore, they also showed rather convincingly that you could reduce GVHD risk, yet, there was a very low risk of relapse in their high-risk patient population. Those were very important [data].
Another study from the University of Minnesota did this with umbilical cord blood. They expanded the regulatory T cells from a third cord blood unit, which is somewhat heroicit is another level of complexity to isolate the cells and then expand them. We did this in matched donorseither matched siblings or matched unrelated donors. We published a paper in JCI Insight several years ago showing the initial results, and they look quite favorable.
Therefore, what I think is most exciting about what Orca Bio has done is they are developing technology to isolate the cells more quickly, to be able to do this on a clinical scale, with precision, and with speed. Also, [they are developing the technology] to be able to distribute it to anybody, because the criticism of all these studies is that, "Oh, that's nice. But, this is a single-institution study. Is this really true? Can this be exported? Could this be something that [an organization] other than these [individual] centers are really focused in this area and have developed these technologies could really do? Orca Bio is developing the technology, and improving the technology, because it's still very cumbersome, and exporting the technology so that you could do this, theoretically, at any center.
That's what I think is most exciting about the Orca Bio abstract; it is demonstrating that this can be done. It certainly opens the door to prevention of GVHD. As we move into an era of using cell-based therapeutics, now, this opens up many other possibilities, because you use these regulatory cells and autoimmune disorders and organ transplant tolerance. There are many other cell types that have potential clinical utility, but getting them, and purifying them, is a big challenge. There are many other possibilities that one could think of.
Obviously, more time will be required to follow these patients, but they certainly are supportive of the idea that you can improve overall outcomes using this strategy. That's what we hope to be able to demonstrate further.
Please focus on the scalability of this approach. Through these types of collaborations, how do you see Orca-T potentially moving through the FDA pipeline?
In academia, we don't develop drugs. It's too much, we don't have the resources, we don't have the capability, and we don't have the monitoring capability that is required for multi-institutional studies. Where these commercial partners come in is, they can raise money for interesting concepts, which Orca Bio has done, and they can export this to other centers, and that's critically important.
As we've seen in the CAR T-cell [therapy] world, that can be a quite successful commercial business. Also going through the process of an FDA approvalwhich Orca Bio is moving along in that processand getting the right designations is critically important to commercial entities. In academia, it's important to us, but that's just not our focus.
We don't have the resources around, the people and the expertise to really drive things through that process. We're good at developing the studies and getting FDA approvals, and [investigational new drug applications], but not really [good at] developing drugs as a commercial entity. This collaboration is key to doing this successfully; for example, at Orca Bio, [they have] technology to separate cells more efficiently and effectively. They also have the resources to do a multi-institutional clinical trial, and the expertise to move something through and present it to the FDA. Those are key components.
Could you expand on the study and respective data from this phase 1/2 trial?
Here at Stanford Cancer Institute, we did find in our patients that giving low doses of immunosuppressive medications with a single agent seem to improve the outcomes, and it's remarkable how well these patients have gone through the transplant. It's a little bit hard to appreciate an abstract until you take care of these patients, and many of them just sort of move to the transplant with relatively little challenges. We have not seen greater risks of things like infection [or] disease recurrence; those are obviously things that will be followed.
When we look at the 1-year GVHD relapse-free survival rate, which is an endpoint that most transplant studies would agree is the most important end point, the overall outcomes are much more favorable compared with a historical control group.
The data are very encouraging, and the overall outcomes look very strong in a reasonable number of patients now. We think it's important for the community to hear about it, and to get it on everybody's radar, and be excited about trying to move this forward as a more standard therapy. This is still a clinical trial, so it's not, it's not part of any standard therapies yet. We are using this quite regularly and have been very encouraged by the ease of which patients go through the transplant. It's still an allogeneic transplant; there still are many challenges there. However, these patients seem to be doing quite well, we're very encouraged, and so we keep going.
How does this approach impact patient outcomes as it relates to quality of life (QoL)?
The hard end points of 1-year relapse-free survival is obviously the most important to patients. However, going through an allogeneic transplant is obviously an incredibly difficult thing. Fortunately, I've only seen it [from] the doctor side, not [as a] patient.
However, I've seen many, many patients, and the quality of their life as they go through this experience is very important to all of us. As we saw these patients go through these studies, we felt like we were capturing something that was really important, and that is the ease [at which] many patients went through this experience, which just seemed different. It's hard to capture that.
It's really important for patients to speak and, and the way patients speak is in different ways. One way is through the QoL measures that they answer. This is [what they find] important, this is what they experiencednot what we say is happening. That's really important to hear that voice too. Those are data we're trying to collect. It's not so easy, because going through a bone marrow transplant is a poor QoL for everybody. But, by just to trying to capture this, [Orca-T seems] better than what we what we thought.
How has this changed the mindset of cell-based approaches in the community?
What has changed is the belief in the concept of cell-based therapies. A lot of these things are somewhat fanciful. It is also important to show that we can translate from an animal model [to a human]. There is a lot of criticism of animal modeling, because people say, "Well, it's nice for animal models, but it doesn't really translate into the clinic." Actually, my view is that because we don't actually follow the animal models, there are many compromises one needs to make. When you translate studies from animals to humans, there are many differences, and it's really important to try to follow them as carefully as you can within the limitations of what is possible. We were very engaged in that and tried to follow as carefully as we could. To me, that is very encouragingthat you can study things in animals that generate new concepts and be able to translate that into a clinical trial.
Obviously, with all of the caveats of an early-phase clinical trial, more time needs to pass, more patients to be treated, and you need to export [the treatment] to other centers. That's a really important point, because there are many things that get lost because, "it's too complicated. It's too expensive. People can't do it." I don't think anybody can do high-speed cell sorting, as a clinical project in a standard or standard cell-processing laboratory. It's above the level of what most processing laboratories can do.
References
Excerpt from:
Negrin Shines Light on the Orca-T Story in GVHD - OncLive
Researchers Trace the Origin of Blood Cancer to Early Childhood, Decades before Diagnosis – PRNewswire
By daniellenierenberg
WASHINGTON, Dec. 8, 2020 /PRNewswire/ --Genetic mutations linked with cancer can occur during childhood or even before birth and proliferate in the body for many years before causing cancer symptoms, according to a new study. The study, which traced the genetic origins of a blood cancer in 10 individuals, suggests there may be untapped opportunities to detect cancer warning signs much earlier and potentially intervene to prevent or slow cancer development.
"Our preliminary findings show these cancer driver mutations were often acquired in childhood, many decades before the cancer diagnosis," said senior study authorJyoti Nangalia, MD,of the Wellcome Sanger Institute and University of Cambridge. "Our results finally answer the common question posed by patients, 'How long has this cancer been growing?' as we were able to study how these particular cancers developed over the entire lifetime of individual patients."
The researchers analyzed bone marrow and blood samples from 10 people with Philadelphia-negative myeloproliferative neoplasms, a type of cancer that causes stem cells in the bone marrow to produce too many blood cells. In the majority of patients, this cancer is driven by a genetic mutation called JAK2V617F. By assessing JAK2V617F, other cancer-linked mutations and hundreds of thousands of other mutations that a person naturally acquires throughout life, the researchers were able to trace the ancestry of different blood cells and estimate the time at which each patient acquired JAK2V617F and other important mutations.
They determined that, in these 10 patients, the first cancer-linked mutations emerged as early as a few weeks after the start of life and up to the first decade of childhood despite clinical disease presenting many decades later in life.
"We were not expecting this," said Dr. Nangalia. "In fact, in one patient, the JAK2 mutation was acquired more than 50 years before their diagnosis."
While it is often assumed that most cancers are diagnosed within a few years of their emergence, the findings point to a more gradual, lifelong process in which a single cell acquires a cancer-linked mutation early in life and then slowly grows over decades, ultimately leading to cancer.
"Some of these cancer-linked mutations are found in healthy individuals as we get older, suggesting that aging causes them," said Dr. Nangalia. "However, aging per se doesn't drive such growth it simply takes a long time for the clones to grow." Sometimes, the growing clones pick up additional cancer-linked mutations along the way, accelerating their growth, researchers found.
"For these patients, we calculated how many of these cancer clones would have been present in the past, and our results suggest that these clones may have been detectable up to 10 to 40 years before diagnosis," said Dr. Nangalia. "In addition to detecting the mutations, the rate at which the mutated clones grew was also very important in determining whether, and when, cancer develops." The findings suggest that genetic testing could help identify people at risk for cancer much earlier than current methods allow, according to researchers.
The next steps would be to understand the factors that influence the different rates of cancer growth and determine whether there could be ways to intervene and slow the growth of cells with cancer-linked mutations. The researchers say their method for pinpointing the origin of this blood cancer could also be applied to other mutations and other blood cancers. "Understanding the timelines of development of different cancers is critical for efforts aimed at early cancer detection and prevention," said Dr. Nangalia.
Jyoti Nangalia, MBBChir,Wellcome Sanger Institute and University of Cambridge, will present this study during the Late-Breaking Abstracts session on Tuesday, December 8 at 7:00 a.m. Pacific time on the ASH annual meeting virtual platform.
For the complete annual meeting program and abstracts, visit http://www.hematology.org/annual-meeting. Follow ASH and #ASH20 on Twitter, Instagram, LinkedIn, and Facebook for the most up-to-date information about the 2020 ASH Annual Meeting.
The American Society of Hematology (ASH) (www.hematology.org) is the world's largest professional society of hematologists dedicated to furthering the understanding, diagnosis, treatment, and prevention of disorders affecting the blood. For more than 60 years, the Society has led the development of hematology as a discipline by promoting research, patient care, education, training, and advocacy in hematology. ASH publishes Blood (www.bloodjournal.org), the most cited peer-reviewed publication in the field, and Blood Advances (www.bloodadvances.org), an online, peer-reviewed open-access journal.
SOURCE American Society of Hematology
See the article here:
Researchers Trace the Origin of Blood Cancer to Early Childhood, Decades before Diagnosis - PRNewswire
Magenta Therapeutics Announces Commencement of First Phase 2 Clinical Trial of MGTA-145 for Stem Cell Mobilization, Oral Presentation of MGTA-145…
By daniellenierenberg
CAMBRIDGE, Mass.--(BUSINESS WIRE)--Magenta Therapeutics (NASDAQ: MGTA), a clinical-stage biotechnology company developing novel medicines to bring the curative power of stem cell transplant to more patients, today announced final clinical results from its earlier completed Phase 1 clinical trial as well as development updates for its MGTA-145 stem cell mobilization therapy, including commencement of enrollment in a Phase 2 clinical trial in multiple myeloma, and its plans for a Phase 2 clinical trial in allogeneic stem cell transplant for patients with acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL) and myelodysplastic syndrome (MDS). The company also previously announced a clinical collaboration with bluebird bio to evaluate MGTA-145 for mobilizing and collecting stem cells in adults and adolescents with sickle cell disease (SCD). Additional preclinical results were also presented at the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition, taking place virtually from December 5-8, 2020, on the Magenta conditioning platform, including MGTA-117 program, which is a targeted antibody-drug conjugate (ADC) to prepare patients for stem cell transplant.
MGTA-145 Advancement to Phase 2 Development in Blood Cancers
The company announced that enrollment has started and is ongoing in a Phase 2 clinical trial of MGTA-145, used in combination with plerixafor, to mobilize and collect stem cells for autologous stem cell transplantation of multiple myeloma patients at Stanford University. Magenta expects that this trial will provide patient-level data on stem cell mobilization and collection, characteristics of the mobilized graft and engraftment in patients with multiple myeloma.
Additionally, through a collaboration with the National Marrow Donor Program/Be The Match, a global leader in facilitating allogeneic hematopoietic stem cell transplantation, Magenta plans to initiate a Phase 2 clinical trial in early 2021 using MGTA-145 to mobilize and collect stem cells from allogeneic donors for transplant in patients with AML, ALL and MDS. Allogeneic stem cell transplant provides a potentially curative therapeutic option for patients with these diseases. This clinical trial will evaluate stem cell mobilization, collection, cell quality, engraftment and the potential for reduced Graft-versus-Host Disease (GvHD), which is of particular importance in the allogeneic transplant setting.
MGTA-145 in Sickle Cell Disease
Magenta Therapeutics recently announced an exclusive clinical collaboration with bluebird bio to evaluate the utility of MGTA-145, in combination with plerixafor, for the mobilization and collection of stem cells in adults and adolescents with SCD.
The data from this clinical trial could provide proof-of-concept for MGTA-145, in combination with plerixafor, as the preferred mobilization regimen for patients with SCD. bluebird bios experience with plerixafor as a mobilization agent in SCD aligns with Magentas combination therapy approach, utilizing MGTA-145 plus plerixafor with potential for safe, rapid and reliable mobilization of sufficient quantities of high-quality stem cells to improve outcomes associated with stem cell transplantation.
MGTA-145 Presentations at ASH
Magenta presented final clinical data from its MGTA-145 stem cell mobilization Phase 1 clinical trial in healthy volunteers at the ASH Annual Meeting. All primary and secondary endpoints were met in the study completed earlier this year.
The results demonstrate that a single dose of MGTA-145, in combination with plerixafor, rapidly and reliably mobilized high numbers of stem cells in a single day without the need for G-CSF for potential use in diseases that can benefit from autologous and/or allogeneic stem cell transplantation. The additional data also offer further confirmation that MGTA-145, in combination with plerixafor, was well tolerated and provides a rapid and reliable method to obtain large numbers of hematopoietic stem cells. Transplant of these cells in preclinical models resulted in enhanced, durable engraftment, in addition to highly immunosuppressive properties, leading to reduced GvHD.
Results from this study provide a robust dataset and proof of concept that MGTA-145, in combination with plerixafor, provides rapid and robust mobilization of stem cells and that these cells have better engraftment potential, are able to be gene modified and engraft and reduce GvHD in preclinical models compared to cells mobilized with other available agents. The data reinforce the availability of compelling opportunities for development in both the autologous and allogeneic transplant settings, said John Davis Jr., M.D., M.P.H., M.S., Head of Research & Development and Chief Medical Officer, Magenta Therapeutics.
The data were presented by Steven M. Devine, MD, Chief Medical Officer of the National Marrow Donor Program/Be The Match and Associate Scientific Director of the CIBMTR (Center for International Blood and Marrow Transplant Research).
Conditioning Program (MGTA-117 and CD45-ADC) Presentations at ASH
Magenta also provided updates on its conditioning platform at the ASH Annual Meeting, including MGTA-117 and CD45-ADC programs. Preclinical data from a study of MGTA-117 demonstrate that it is an effective, potent conditioning agent for transplant with anti-leukemic activity, significantly decreasing tumor burdens, leading to delayed tumor growth and increased median survival rates in animal models of AML. Ongoing GLP toxicology and GMP manufacturing progress continue to be supportive of advancing MGTA-117 towards an IND filing in AML and MDS.
Additionally, preclinical data from a study of Magentas CD45-ADC, a CD45-targeted conditioning agent designed to remove the cells that cause autoimmune diseases to enable curative immune reset, demonstrated the ability to achieve successful outcomes as a single agent in the most challenging disease model through fully mismatched allogeneic hematopoietic stem cell transplant, where only radiation or combinations of toxic chemotherapies are available, potentially providing patients the option of a reduced toxicity conditioning regimen. The company continues to evaluate this program preclinically.
About MGTA-145
MGTA-145 is being developed in combination with plerixafor to harness complementary chemokine mechanisms to mobilize hematopoietic stem cells for collection and transplantation. This new combination has the potential to be the preferred mobilization regimen for rapid and reliable mobilization and collection of hematopoietic stem cells to improve outcomes in autologous and allogeneic stem cell transplantation, which can rebuild a healthy immune system for patients with blood cancers, genetic diseases and autoimmune disorders.
MGTA-145 has the potential to replace the current standard of care for patients and allogeneic donors who currently rely on the use of granulocyte-colony stimulating factor (G-CSF) alone or in combination with plerixafor, which can take up to five days or longer to mobilize sufficient numbers of stem cells, often resulting in significant bone pain and other side effects.
About Magenta Therapeutics
Magenta Therapeutics is a clinical-stage biotechnology company developing medicines to bring the curative power of immune system reset through stem cell transplant to more patients with blood cancer, genetic diseases and autoimmune diseases. Magenta is combining leadership in stem cell biology and biotherapeutics development with clinical and regulatory expertise, a unique business model and broad networks in the stem cell transplant world to revolutionize immune reset for more patients.
Magenta is based in Cambridge, Mass. For more information, please visit http://www.magentatx.com.
Follow Magenta on Twitter: @magentatx.
Forward-Looking Statement
This press release may contain forward-looking statements and information within the meaning of The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as may, will, could, should, expects, intends, plans, anticipates, believes, estimates, predicts, projects, seeks, endeavor, potential, continue or the negative of such words or other similar expressions can be used to identify forward-looking statements. The express or implied forward-looking statements included in this press release are only predictions and are subject to a number of risks, uncertainties and assumptions, including, without limitation risks set forth under the caption Risk Factors in Magentas Annual Report on Form 10-K filed on March 3, 2020, as updated by Magentas most recent Quarterly Report on Form 10-Q and its other filings with the Securities and Exchange Commission. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this press release may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. You should not rely upon forward-looking statements as predictions of future events. Although Magenta believes that the expectations reflected in the forward-looking statements are reasonable, it cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, except as required by law, neither Magenta nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements included in this press release. Any forward-looking statement included in this press release speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law.
Haywards Heath woman’s bid to fund stem cell treatment to combat MS – Mid Sussex Times
By daniellenierenberg
Joceline Colvert was diagnosed with relapsing remitting Multiple Sclerosis in her early 20s and says she spent the first eight years researching and managing her condition while trying to mention it as little as possible to others and completing her Sound Production degree.
I spent most of my late 20s and early 30s finding ways to manage relapses, the symptoms of which have included whole body numbness, loss of the use of both hands, right eye blindness, vertigo and double vision, she said. Thankfully these symptoms did resolve however left scarring on my nerves. This results in reduced vision in my formerly blind eye and hands that dont function very well with repetitive tasks.
This semi-denial worked for me until about 2010 when I started to become a bit limpy which I did my best to hide. After a couple of memorable falls and fractures I decided to face up to being slightly rickety and got a hiking pole that I used occasionally in public. Since then Ive needed to get used to being visibly disabled, and switch between two hiking poles for very short distances and a wheelchair everywhere else.
Joceline, who lives with her husband and her five beloved cats and dogs, says she is not eligible for Haematopoietic Stem Cell Transplantation (HSCT), on the NHS which is the first treatment I have ever got excited about and believe could work. It could be truly life-changing.
As a result she is trying to raise money to fund the treatment herself.
HSCT is a procedure that aims to reset the faulty immune system which, in my case, is attacking my nervous system from within, Joceline said. Stem cells will be taken from my bone marrow or blood before my immune system is wiped out with chemotherapy. My cells are then reintroduced into my blood, where they grow a new immune system which will hopefully no longer attack my nerves or have any memory of MS.
The aim of HSCT is to completely halt progression, putting MS into remission with no requirement for immunosuppressant drug therapy. The success rate for relapsing remitting MS is 80% - 90% which is absolutely phenomenal compared to the limited available drug treatments, which only aim to slow down disability.
HSCT is available on the NHS, however there is a very strict criteria for which I do not qualify. The expense of the treatment and the increased pressures on the public purse mean the NHS will only treat patients who have been diagnosed for fewer than 15 years.
I have been diagnosed for 18 years.
I had prepared myself for this possibility and, for the last year, have been researching treatment with The National Pirogov Medical Centre Russia (Moscow). Russia has been pioneering in their use of HSCT to treat MS and are world renowned for their expertise and care. Im excited to have a treatment date in March 2021 which fills me with hope for a future free from progression. I need your help to get there.
Joceline, who loves making stop-motion animation puppets and props and playing musical instruments, says the treatment will cost 40,800, and the flights 800.
She has launched a Go Fund Me page at https://gf.me/u/y538k2 which has already seen donations of more than 26,000.
I am incredibly grateful for any help you can give towards enabling me to access this life-changing treatment, she said.
After almost two decades of managing MS flare-ups and their consequences, its hard to put into words just what a future without them would mean to me.
Thank you for reading this and for any help you can put towards this goal.
See the original post:
Haywards Heath woman's bid to fund stem cell treatment to combat MS - Mid Sussex Times
Antileukemic Activity Seen With Flotetuzumab in Primary Induction Failure, Early-Relapse AML – Hematology Advisor
By daniellenierenberg
Flotetuzumab was found to demonstrate antileukemic activity in patients with primary induction failure (PIF) and early-relapse acute myeloid leukemia (ER-AML), and the treatment appears tolerable with infrequent neurologic adverse events, according to results from an updated analysis of an ongoing open-label phase 1/2 study (ClinicalTrials.gov Identifier: NCT02152956). The preliminary findings were presented by Ibrahim Aldoss, MD, of the Gehr Family Center for Leukemia Research at City of Hope in Duarte, California, at the virtual 62nd American Society of Hematology (ASH) Annual Meeting and Exposition.
CD123 is overexpressed on AML cells, including leukemia stem cells, as well as other hematological malignancies, said Dr Aldoss. Flotetuzumab is a humanized CD3 x CD123 bispecific T-cell engager that redirects T cells to kill tumor cells expressing CD123.
The open-label, single-arm, multicenter, phase 1/2 study previously identified the recommended phase 2 dosage of flotetuzumab as 500 ng/kg/d administered via continuous infusion in 28-day cycles following a step-up lead-in dose administered during cycle 1 in week 1 of treatment. The primary objective of the study was to assess safety and antileukemic activity of flotetuzumab in patients with PIF/ER-AML.
A total of 44 patients (PIF, n= 27; ER-AML, n=17) were included in the study. Median patient age was 63.5 years (range, 28.0-81.0), and most patient were men (70.5%). According to the European LeukemiaNet (ELN) 2017 risk stratification criteria, the majority of patients had nonfavorable risk (97.7%).
Evidence of antileukemic activity was documented in 59.1% of patients, with a median decrease of 81.0% in bone marrow blasts. Median time to first response was 1 cycle (range, 1-3).
The combined complete response rate (CR, <5% bone marrow blast) and CR with partial hematologic recovery (CRh) was 25.0% (PIF, 33.3%; ER-AML, 11.8%) and 31.8% when including CR with incomplete hematologic recovery (CRi). Among the 14 patients with CR/CRh/CRi, 8 patients subsequently underwent stem cell transplantation.
In addition, morphologic leukemia-free state was reported in 3 patients (PIF, n=1; ER-AML, n=2). Of the 10 patients with TP53 mutation, 5 were reported to have CRR/CRh/CRi, and 3 of those patients (60.0%) underwent stem cell transplantation.
For all patients who achieved CR/CRh/CRi, median duration of response was 8.13 months, and median overall survival was 10.7 months.
Cytokine release syndrome (CRS), the most frequently reported treatment-related adverse event, occurred in 100% of patients (n=44; all grade). One grade 3 CRS event occurred. Approximately half of CRS events (52%) occurred during step-up dosing in the first week of treatment, and the incidence of CRS progressively decreased over time.
Neurologic adverse events were reported as infrequent and of mild to moderate severity (all-grade headache, n=13; 29.5%). Neurologic treatment-related adverse events of grade 3 or more were confusional state (n=3) and dizziness (n=1).
Flotetuzumab demonstrated encouraging activity in patients with primary induction failure in early-relapse AML, a population with poor prognosis and high unmet medical need, Dr Aldoss concluded.
The study (ClinicalTrials.gov Identifier: NCT02152956) is currently enrolling patients.
Disclosure: Some authors have declared affiliations with or received funding from the pharmaceutical industry. Please refer to the original study for a full list of disclosures.
Read more ofHematology Advisorscoverage of the ASH 2020 meeting by visiting theconference page.
Aldoss I, Uy G, Vey N, et al. Flotetuzumab as salvage therapy for primary induction failure and early relapse acute myeloid leukemia. Presented at: American Society of Hematology (ASH) 62nd Annual Meeting and Exposition; December 5-8, 2020. Abstract 331.
Venetoclax/Azacitidine Combination Efficacious for the Treatment of Older Patients With Higher-Risk Myelodysplastic Syndrome – Oncology Nurse Advisor
By daniellenierenberg
The following article features coverage from the ASH 2020 virtual meeting. Click here to read more of Oncology Nurse Advisors conference coverage.
Patients who received venetoclax with azacytidine for the treatment of higher-risk myelodysplastic syndrome (HR-MDS) had high overall survival rates and clinically meaningful improvements of dyspnea and fatigue through 48 weeks. These findings were presented during the American Society of Hematology (ASH) 62nd Annual Meeting and Exposition.
Jacqueline S. Garcia, MD, coauthor of this study, explained the mechanism of this therapy. Apoptosis is normally under tight control by the interaction between pro-survival and pro-biotic proteins. In HR-MDS, myeloblasts overexpress BCL-2 and blasts are generally highly prone to pro-apoptotic proteins. Azacytidine indirectly decreases other apoptotic proteins, which sensitizes cells to venetoclax. Venetoclax is a BCL-2 inhibitor, which induces death. Thus, these drugs have the potential to irreversibly commit the cell to death.
Patients (N=78) with HR-MDS who were not candidates for intensive chemotherapy were recruited for this ongoing, open-label, dose-escalation, phase 1b study. Study participants received venetoclax 400 or 800 mg for 28 days followed by an escalating dose (100, 200, and 400 mg) for 14 days in a 28-day cycle with azacitidine 75 mg/m2 subcutaneously or intravenously administered on the first 7 days of each cycle. Participants were assessed for adverse events and efficacy.
Patient group was 75% men, median age 71 years (range, 26 to 85) and 56% had very high-risk disease.
Of the 31 patients with baseline marrow data, the most frequent mutations were located in tumor protein p53 (TP53; 35.5%), additional sex combs like 1 (ASXL1; 19.4%), and stromal antigen 2 (STAG2; 16.1%).
All participants experienced at least 1 adverse event during the study. The most commonly observed events were constipation (54%), nausea (55%), and neutropenia (83%). Adverse events grade 3 or higher were experienced by 96% of patients and included febrile neutropenia (49%) and thrombocytopenia (42%). Few infections were observed, likely due to the antibiotic prophylaxis.
At 30 days, the mortality rate was 1% and 1.3% experienced disease progression. A total of 16 patients received post-study transplants (bone marrow, 7 patients; stem cell, 9 patients).
The objective response rate was 79%; in which 39.7% entered into complete remission, 39.7% into marrow complete remission, and 14.1% had stable disease.
The median duration of response was 12.9 months (range, 12.1 to 16.8), and among those who achieved complete remission, the median duration of response after remission was 13.8 months (range, 6.5 to 20.9). The median time to complete remission was 2.6 months (range, 1.2 to 19.6).
Physical function through 48 weeks was generally maintained and fatigue, dyspnea, and global health quality of life were improved among patients who received 400 mg of venetoclax for 14 days.
This study was limited by its small sample size and short duration; however, this study was still on-going, and a phase 3 trial has begun.
These results indicated venetoclax with azacitidine was efficacious, allowing for maintenance of physical functioning for up to 48 weeks among patients with HR-MDS who were not candidates for intensive chemotherapy.
Disclosure: Multiple authors declared affiliations with industry. Please refer to the original article for a full list of disclosures.
Reference
Garcia JS, Wei AH, Borate U, et al. Safety, efficacy, and patient-reported outcomes of venetoclax in combination with azacitidine for the treatment of patients with higher-risk myelodysplastic syndrome: a phase 1b study. Presented at: American Society of Hematology (ASH) 62nd Annual Meeting and Exposition; December 5-8, 2020. Abstr 656.
Rocket Pharmaceuticals Presents Positive Clinical Data from its Fanconi Anemia and Leukocyte Adhesion Deficiency-I Programs at the 62nd American…
By daniellenierenberg
NEW YORK--(BUSINESS WIRE)--Rocket Pharmaceuticals, Inc. (NASDAQ: RCKT) (Rocket), a clinical-stage company advancing an integrated and sustainable pipeline of genetic therapies for rare childhood disorders, today presents updated interim data from its Fanconi Anemia (FA) and Leukocyte Adhesion Deficiency-I (LAD-I) programs at the 62nd American Society of Hematology (ASH) Annual Meeting. The data are highlighted in two oral presentations.
We are highly pleased with the data presented at ASH demonstrating ongoing evidence of efficacy and durability using Process B in both FA and LAD-I as we move towards potential registration, said Gaurav Shah, M.D., Chief Executive Officer and President of Rocket. Follow-up data from the Phase 1 and 2 trials for FA continue to support RP-L102 as a potential hematologic treatment option in the absence of cytotoxic conditioning. In five of the seven patients treated as of October 2020, there was evidence of engraftment. In addition, stabilization of peripheral blood counts in two of the three patients with at least 12-month follow-up, which declined substantially in these patients prior to gene therapy, suggests a halt in bone marrow failure progression. We look forward to reporting longer-term follow-up on these patients in the first half of 2021.
Dr. Shah continued, Additionally, we continue to see encouraging evidence of efficacy for RP-L201 for the treatment of LAD-I. Patients have shown sustained CD18 expression of 23% to 40%, far exceeding the 4-10% threshold associated with survival into adulthood. These data, on top of our exciting results from our lentiviral program for PKD, show our steady progress across three of our five gene therapy programs. We are proud of this progress and are committed to advancing our investigational gene therapies through development for patients and families facing these devastating disorders.
Key findings and details for each presentation are highlighted below. To access the presentations at the conclusion of the oral presentation, please visit: https://www.rocketpharma.com/ash-presentations/
Gene Therapy for Fanconi Anemia, Complementation Group A: Updated Results from Ongoing Global Clinical Studies of RP-L102The data presented in the oral presentation are from seven of the nine patients treated as of the cutoff date of October 2020 in both the U.S. Phase 1 and global Phase 2 studies of RP-L102 for FA. Seven patients had follow-up data of at least 2-months, and three of the seven patients had been followed for 12-months or longer. Key highlights from the presentation include:
Presentation Details:Title: Gene Therapy for Fanconi Anemia, Complementation Group A: Updated Results from Ongoing Global Clinical Studies of RP-L102Session Title: Gene Editing, Therapy and Transfer IPresenter: Agnieszka Czechowicz, M.D., Ph.D., Assistant Professor of Pediatrics, Division of Stem Cell Transplantation, Stanford University School of MedicineSession Date: Monday, December 7, 2020Session Time: 11:30 a.m. - 1:00 p.m. (Pacific Time)Presentation Time: 12:15 p.m. (Pacific Time)
Phase 1/2 Study of Lentiviral-Mediated Ex-Vivo Gene Therapy for Pediatric Patients with Severe Leukocyte Adhesion Deficiency-I (LAD-I): Results from Phase 1The data presented in the oral presentation are from three pediatric patients with severe LAD-I, as defined by CD18 expression of less than 2%. The patients were treated with RP-L201, Rockets ex-vivo lentiviral gene therapy candidate. Patient L201-003-1001 was 9-years of age at enrollment and had been followed for 12-months as of a cutoff date of November 2020. Patient L201-003-1004 was 3-years of age at enrollment and had been followed for over 6-months. Patient L201-003-2006 was 7-months of age at enrollment and was recently treated with RP-L201. Key highlights from the presentation include:
Rockets LAD-I research is made possible by a grant from the California Institute for Regenerative Medicine (Grant Number CLIN2-11480). The contents of this press release are solely the responsibility of Rocket and do not necessarily represent the official views of CIRM or any other agency of the State of California.
Presentation Details:Title: Phase 1/2 Study of Lentiviral-Mediated Ex-Vivo Gene Therapy for Pediatric Patients with Severe Leukocyte Adhesion Deficiency-I (LAD-I): Results from Phase 1Session Title: Gene Editing, Therapy and Transfer IPresenter: Donald Kohn, M.D., Professor of Microbiology, Immunology and Molecular Genetics, Pediatrics (Hematology/Oncology), Molecular and Medical Pharmacology, and member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at the University of California, Los AngelesSession Date: Monday, December 7, 2020Session Time: 11:30 a.m. - 1:00 p.m. (Pacific Time)Presentation Time: 12:30 p.m. (Pacific Time)
Conference Call DetailsRocket management will host a conference call and webcast today December 7, at 6:00 p.m. EST. To access the call and webcast, please click here. The webcast replay will be available on the Rocket website following the completion of the call.
Investors may listen to the call by dialing (866) 866-1333 from locations in the United States or +1 (404) 260-1421 from outside the United States. Please refer to conference ID number 50038102
About Fanconi AnemiaFanconi Anemia (FA) is a rare pediatric disease characterized by bone marrow failure, malformations and cancer predisposition. The primary cause of death among patients with FA is bone marrow failure, which typically occurs during the first decade of life. Allogeneic hematopoietic stem cell transplantation (HSCT), when available, corrects the hematologic component of FA, but requires myeloablative conditioning. Graft-versus-host disease, a known complication of allogeneic HSCT, is associated with an increased risk of solid tumors, mainly squamous cell carcinomas of the head and neck region. Approximately 60-70% of patients with FA have a Fanconi Anemia complementation group A (FANCA) gene mutation, which encodes for a protein essential for DNA repair. Mutation in the FANCA gene leads to chromosomal breakage and increased sensitivity to oxidative and environmental stress. Increased sensitivity to DNA-alkylating agents such as mitomycin-C (MMC) or diepoxybutane (DEB) is a gold standard test for FA diagnosis. Somatic mosaicism occurs when there is a spontaneous correction of the mutated gene that can lead to stabilization or correction of a FA patients blood counts in the absence of any administered therapy. Somatic mosaicism, often referred to as natural gene therapy provides a strong rationale for the development of FA gene therapy because of the selective growth advantage of gene-corrected hematopoietic stem cells over FA cells.
About Leukocyte Adhesion Deficiency-ISevere Leukocyte Adhesion Deficiency-I (LAD-I) is a rare, autosomal recessive pediatric disease caused by mutations in the ITGB2 gene encoding for the beta-2 integrin component CD18. CD18 is a key protein that facilitates leukocyte adhesion and extravasation from blood vessels to combat infections. As a result, children with severe LAD-I are often affected immediately after birth. During infancy, they suffer from recurrent life-threatening bacterial and fungal infections that respond poorly to antibiotics and require frequent hospitalizations. Children who survive infancy experience recurrent severe infections including pneumonia, gingival ulcers, necrotic skin ulcers, and septicemia. Without a successful bone marrow transplant, mortality in patients with severe LAD-I is 60-75% prior to the age of 2 and survival beyond the age of 5 is uncommon. There is a high unmet medical need for patients with severe LAD-I.
About Rocket Pharmaceuticals, Inc.Rocket Pharmaceuticals, Inc. (NASDAQ: RCKT) (Rocket) is advancing an integrated and sustainable pipeline of genetic therapies that correct the root cause of complex and rare childhood disorders. The companys platform-agnostic approach enables it to design the best therapy for each indication, creating potentially transformative options for patients afflicted with rare genetic diseases. Rocket's clinical programs using lentiviral vector (LVV)-based gene therapy are for the treatment of Fanconi Anemia (FA), a difficult to treat genetic disease that leads to bone marrow failure and potentially cancer, Leukocyte Adhesion Deficiency-I (LAD-I), a severe pediatric genetic disorder that causes recurrent and life-threatening infections which are frequently fatal, Pyruvate Kinase Deficiency (PKD) a rare, monogenic red blood cell disorder resulting in increased red cell destruction and mild to life-threatening anemia and Infantile Malignant Osteopetrosis (IMO), a bone marrow-derived disorder. Rockets first clinical program using adeno-associated virus (AAV)-based gene therapy is for Danon disease, a devastating, pediatric heart failure condition. For more information about Rocket, please visit http://www.rocketpharma.com.
Rocket Cautionary Statement Regarding Forward-Looking StatementsVarious statements in this release concerning Rocket's future expectations, plans and prospects, including without limitation, Rocket's expectations regarding its guidance for 2020 in light of COVID-19, the safety, effectiveness and timing of product candidates that Rocket may develop, to treat Fanconi Anemia (FA), Leukocyte Adhesion Deficiency-I (LAD-I), Pyruvate Kinase Deficiency (PKD), Infantile Malignant Osteopetrosis (IMO) and Danon Disease, and the safety, effectiveness and timing of related pre-clinical studies and clinical trials, may constitute forward-looking statements for the purposes of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995 and other federal securities laws and are subject to substantial risks, uncertainties and assumptions. You should not place reliance on these forward-looking statements, which often include words such as "believe," "expect," "anticipate," "intend," "plan," "will give," "estimate," "seek," "will," "may," "suggest" or similar terms, variations of such terms or the negative of those terms. Although Rocket believes that the expectations reflected in the forward-looking statements are reasonable, Rocket cannot guarantee such outcomes. Actual results may differ materially from those indicated by these forward-looking statements as a result of various important factors, including, without limitation, Rocket's ability to monitor the impact of COVID-19 on its business operations and take steps to ensure the safety of patients, families and employees, the interest from patients and families for participation in each of Rockets ongoing trials, our expectations regarding the delays and impact of COVID-19 on clinical sites, patient enrollment, trial timelines and data readouts, our expectations regarding our drug supply for our ongoing and anticipated trials, actions of regulatory agencies, which may affect the initiation, timing and progress of pre-clinical studies and clinical trials of its product candidates, Rocket's dependence on third parties for development, manufacture, marketing, sales and distribution of product candidates, the outcome of litigation, and unexpected expenditures, as well as those risks more fully discussed in the section entitled "Risk Factors" in Rocket's Quarterly Report on Form 10-Q for the quarter ended September 30, 2020, filed November 6, 2020 with the SEC. Accordingly, you should not place undue reliance on these forward-looking statements. All such statements speak only as of the date made, and Rocket undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
Precigen Presents New Data Supporting the Safety, Clinical Activity, Expansion and Persistence of PRGN-3006 UltraCAR-T at the 62nd ASH Annual Meeting…
By daniellenierenberg
GERMANTOWN, Md., Dec. 7, 2020 /PRNewswire/ -- Precigen Inc., a biopharmaceutical company specializing in the development of innovative gene and cell therapies to improve the lives of patients, today announced at the 62nd ASH Annual Meeting and Exposition (Abstract 2864) clinical progress and new data from the ongoing Phase 1/1b clinical study of PRGN-3006UltraCAR-Tin patients with relapsed or refractory (r/r) acute myeloid leukemia (AML) and higher risk myelodysplastic syndrome (MDS) (clinical trial identifier: NCT03927261).
AML is a rapidly progressing disease with poor prognosis and high unmet need. Precigen's UltraCAR-T platform is designed to overcome limitations of currently available chimeric antigen receptor (CAR)-T therapies by utilizing an advanced overnight non-viral gene delivery manufacturing process at a medical center's cGMP facility without the need for ex vivo expansion. Current CAR-T cell therapies are limited due to, inter alia, the prolonged interval between apheresis to product infusion and an exhausted phenotype of T cells resulting from lengthy ex vivo expansion. As announced in November 2020, UltraCAR-T cells for the PRGN-3006 study are now manufacturedovernight using Precigen's proprietary UltraPorator device. PRGN-3006 UltraCAR-T is a multigenic autologous CAR-T simultaneously expressing a CAR specifically targeting CD33; membrane bound IL-15 (mbIL15) for enhanced in vivo expansion and persistence; and a kill switch to conditionally eliminate CAR-T cells for an improved safety profile. CD33 is over-expressed on AML blasts with lesser expression on normal hematopoietic stem cells.
An investigator-initiated, non-randomized Phase 1/1b dose-escalation study to evaluate the safety and maximal tolerated dose of PRGN-3006 UltraCAR-T is currently ongoing in collaboration with the H. Lee Moffitt Cancer Center & Research Institute (Moffitt). The study population includes adult patients ( 18 years) with r/r AML and hypomethylating agent (HMA) failure, higher risk MDS or chronic myelomonocytic leukemia (CMML) patients with 5% blasts. To test the hypothesis that expression of mbIL15 on PRGN-3006 can promote UltraCAR-T cell expansion and persistence without the need for lymphodepletion and improve the overall safety profile, studysubjects receive the PRGN-3006 infusion either without prior lymphodepletion (Cohort 1) or following lymphodepleting chemotherapy (Cohort 2). A multicenter expansion of the trial is planned.
Key findings:
A case study of the patient with the longest follow-up as of the data cutoff was also presented. This patient received, one day after gene transfer and without prior lymphodepletion, a very low dose, approximately three hundred thousand UltraCAR-T per kilogram (3 x 105 UltraCAR-T/kg) for a total of only 24 million UltraCAR-T. She is a 69 year old female with secondary AML (sAML) and four prior lines of therapy, including induction chemotherapy (IC), allogenic hematopoietic stem cell transplantation (allo-HSCT), HMA plus venetoclax (HMA+VEN), refractory to all therapy post allo-HSCT. The patient had approximately 40% peripheral blasts and 47% bone marrow blasts at baseline.
Case study findings:
"There is an urgent need for novel therapies for relapsed or refractory AML patients as the median overall survival for this patient population is less than six months. Current CAR-T approaches for AML have faced challenges due to long manufacturing durations resulting in subsequent delays in treatment," said David A. Sallman, MD, of Moffitt and lead investigator for the PRGN-3006 clinical study. "We are encouraged by the initial data, including safety and manufacturing success from patients treated with autologous UltraCAR-T cells, which were manufactured on-site with almost instant turnaround. We are excited by the expansion and continued persistence of PRGN-3006 UltraCAR-T cells in the patient case study for over seven months post-infusion without prior lymphodepletion and are looking forward to higher doses in the lymphodepleted and non-lymphodepletion cohorts."
"Currently commercialized CAR-T therapies have not demonstrated the persistence needed to drive sustained, durable responses," said Helen Sabzevari, PhD, President and CEO of Precigen. "The results from Dr. Sallman's patient case study are particularly encouraging as the patient received a very low dose of cells without any ex vivo expansion or activation and no lymphodepletion, which highlights the importance of membrane bound IL-15 in expansion and persistence of these cells and, we believe, differentiates the UltraCAR-T platform from other CAR-T's. In particular, expansion and persistence of UltraCAR-T cells in the patient's blood through seven months post-infusion show promise for the durability of PRGN-3006. We look forward to providing additional details for the PRGN-3006 study at our upcoming clinical update call this month."
About Acute Myeloid Leukemia (AML)AML is a cancer that starts in the bone marrow, but most often moves into the blood.1 Though consideredrare, AML is among the most common types of leukemia in adults.2 In 2019, it was estimated that 21,450 new cases of AML would be diagnosed in the US.2 AML is uncommon before the age of 45 and the average age of diagnosis is about 68.2 The prognosis for patients with AML is poor with an average 5year survival rate of approximately 25 percent overall, and less than a 5 percent 5year survival rate for patients older than 65.3 Amongst elderly AML patients ( 65 years of age), median survival isshort, ranging from 3.5 months for patients 65 to 74 years of age to 1.4 months for patients 85 years of age.3
About Myelodysplastic Syndrome (MDS)MDS are diseases of the bone marrow generally found in adults in their 70s.4 Incidence in the US is not known for sure, but estimates range from 10,000 each year and higher.4 Using International Prognostic Scoring System (IPSS-R), median survival for MDS patients can vary from less than one year for the "very high" IPSS-R risk group to more than eight years for the "very low" IPSS-R group.4
About PRGN-3006 UltraCAR-TPRGN-3006 UltraCAR-T is a multigenic autologous CAR-T cell treatment utilizing Precigen's non-viral Sleeping Beauty system to simultaneously express a CAR specifically targeting CD33, which is over expressed on acute myeloid leukemia blasts with lesser expression on normal hematopoietic stem cell populations and minimal non-hematopoietic expression; membrane bound IL-15 for enhanced in vivo expansion and persistence; and a kill switch to conditionally eliminate CAR-T cells for animproved safety profile. PRGN-3006 is being evaluated in collaboration with the Moffitt Cancer Center in a nonrandomized, investigatorinitiated Phase 1/1b dose escalation study to evaluate the safety and maximal tolerated dose of PRGN3006 UltraCAR-T (clinical trial identifier: NCT03927261). The study population includes patients with relapsed or refractory acute myeloid leukemia or higher risk myelodysplastic syndrome. The US Food and Drug Administration (FDA) has granted orphan drug designation (ODD) for PRGN-3006 UltraCAR-T in patients with AML.
Precigen: Advancing Medicine with PrecisionPrecigen (Nasdaq: PGEN) is a dedicated discovery and clinical stage biopharmaceutical company advancing the next generation of gene and cell therapies using precision technology to target urgent and intractable diseases in our core therapeutic areas of immuno-oncology, autoimmune disorders, and infectious diseases. Our technologies enable us to find innovative solutions for affordable biotherapeutics in a controlled manner. Precigen operates as an innovation engine progressing a preclinical and clinical pipeline of well-differentiated unique therapies toward clinical proof-of-concept and commercialization. For more information about Precigen, visit http://www.precigen.com or follow us on Twitter @Precigen and LinkedIn.
TrademarksPrecigen, UltraCAR-T, UltraPorator and Advancing Medicine with Precision are trademarks of Precigen and/or its affiliates. Other names may be trademarks of their respective owners.
Cautionary Statement Regarding Forward-Looking StatementsSome of the statements made in this press release are forward-looking statements. These forward-looking statements are based upon the Company's current expectations and projections about future events and generally relate to plans, objectives, and expectations for the development of the Company's business, including the timing and progress of preclinical studies, clinical trials, discovery programs and related milestones, the promise of the Company's portfolio of therapies, and in particular its CAR-T therapies, and the Company's refocus to a healthcare-oriented business. Although management believes that the plans and objectives reflected in or suggested by these forward-looking statements are reasonable, all forward-looking statements involve risks and uncertainties, including the possibility that the timeline for the Company's clinical trials might be impacted by the COVID-19 pandemic, and actual future results may be materially different from the plans, objectives and expectations expressed in this press release. The Company has no obligation to provide any updates to these forward-looking statements even if its expectations change. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. For further information on potential risks and uncertainties, and other important factors, any of which could cause the Company's actual results to differ from those contained in the forward-looking statements, see the section entitled "Risk Factors" in the Company's most recent Annual Report on Form 10-K and subsequent reports filed with the Securities and Exchange Commission.
References1 American Cancer Society. What is Acute Myeloid Leukemia (AML)?2 American Cancer Society. Key Statistics for Acute Myeloid Leukemia (AML)3 Thein, M., et al., Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over 3 decades. Cancer, 2013. 119(15): p.2720-74 American Cancer Society.Key Statistics for Myelodysplastic Syndromes
For more information, contact:
View original content to download multimedia:http://www.prnewswire.com/news-releases/precigen-presents-new-data-supporting-the-safety-clinical-activity-expansion-and-persistence-of--prgn-3006-ultracar-t-at-the-62nd-ash-annual-meeting-and-exposition-301186957.html
SOURCE Precigen, Inc.
Read the rest here:
Precigen Presents New Data Supporting the Safety, Clinical Activity, Expansion and Persistence of PRGN-3006 UltraCAR-T at the 62nd ASH Annual Meeting...
CLL patients in England to get AZ’s Calquence after okay from NICE – – pharmaphorum
By daniellenierenberg
NHS England is to grant immediate access to AstraZenecas cancer drug Calquence (acalabrutinib) for certain patients with chronic lymphocytic leukaemia (CLL) after NICE backed it in first draft recommendations.
NICE recommended regular NHS funding for Calquence in CLL who are considered high-risk due to 17p deletion or TP53 mutations.
It is also recommended for adults with CLL who have had at least one previous treatment and only if AbbVie and Janssens class rival Imbruvica (ibrutinib) is their only suitable treatment option.
NHS England is granting access via an interim funding arrangement with AstraZeneca, which will end 30 days after publication of positive final guidance, after which treatment will be funded by routine commissioning budgets.
However the guidance has rejected Calquence for a third group of patients with untreated, non-high risk CLL who are unsuitable for treatment with chemotherapy.
AZ said it will provide further data analyses for continued discussions with NICE about this group of patients.
Calquence was approved in CLL by the EMA last month as monotherapy or in combination with Roches Gazyvaro (obinutuzumab).
In CLL, too many blood stem cells in the bone marrow become abnormal white blood cells, and these have difficulty in fighting infections.
As the number of abnormal cells grows there is less room for healthy white blood cells, red blood cells, and platelets. This could result in anaemia, infection, and bleeding.
B-cell receptor signalling through Brutons tyrosine kinase (BTK) is one of the essential growth pathways for CLL.
In B-cells, BTK signalling results in the activation of pathways necessary for growth: proliferation, trafficking, chemotaxis, and adhesion.
Calquence binds selectively to BTK, inhibiting its activity.
This is the second recommendation of a therapy for CLL in the space of a month in November it recommended AbbVie/Roches chemotherapy-free option of Venclyxto (venetoclax) and Gazyva.
NICEs decision allows for a 12-month fixed duration treatment option based on data from the phase 3 CLL14 trial.
Read more:
CLL patients in England to get AZ's Calquence after okay from NICE - - pharmaphorum
Regenerative Medicine: Market Trends and Legal Developments on the Horizon for 2021 – MedTech Intelligence
By daniellenierenberg
As the second wave of the pandemic engulfs us and the world works at warp speed to develop vaccines and therapies to respond, the importance of regenerative medicine has never been higher. Since 2017, Goldman Sachs has touted the sector as one of the most compelling areas for venture capital investment. With billions of dollars of global government spending being poured into the search for vaccines and therapies to respond to the novel coronavirus, and with the FDA having now granted approval to the first vaccines based on CRISPR mRNA gene-editing technologies, business models based on regenerative medicines are commanding record values. Despite the flood of cash into regenerative medicine, legal and ethical considerations will continue to cause much controversy.
Regenerative medicine ultimately accelerates the human bodys healing process. It is an area of biomedical sciences that involves medical treatments to repair or replace damaged cells, tissues, or organs. Instead of merely focusing on the symptoms, regenerative medicine uses cellular therapies, tissue engineering, medical devices, and artificial organs to improve peoples health. For example, stem cell therapies, tissue grafts, and organ transplants are all part of regenerative medicine.
Today, cellular and acellular regenerative medicines are often used in clinical procedures such as cell, immunomodulation, and tissue engineering therapies. They have the potential to effectively treat many chronic diseases, including Alzheimers, Parkinsons and cardiovascular disorders, osteoporosis, and spinal cord injuries.
A small number of unscrupulous actors, according to the FDA, however, have seized on the clinical promise of regenerative medicine to offer patients unproven treatments. The FDA and other regulators are challenged to provide assurances of safety for these therapies without stifling development, as well as to approve treatments based on manipulation of stem cells derived from human and animal embryos given the ethical issues involved.
In the future, stem cell research will play an increasingly outsized role in regenerative medicine techniques. In November 2020, voters in California narrowly passed Proposition 14, a referendum to approve $5.5 billion in new government funding for stem cell research. Other governments around the world are doing the same.
Today, the growing prevalence of chronic medical ailments and genetic disorders across the globe is a primary factor driving the regenerative medicine industrys growth, according to the Regenerative Medicine Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2020-2025. The increasing aging population, prone to various musculoskeletal, oncological, dermatological, and cardiological disorders, is a key growth driver. Widespread adoption of organ transplantation is another contributing factor to this growth in market share. The current pandemic that began in January 2020, however, has changed the paradigm for regenerative medicine.
Market applications are burgeoning. Regenerative medicine can prevent and cure disease through effective vaccines and efficacious therapies. It can minimize the risk of organ rejection post-transplant and speed recovery. Technological advancements in cell-based therapies, such as the development of 3-D bioprinting techniques and the adoption of artificial intelligence in the production of regenerative medicines, are also stimulating growth. These advancements also facilitate dermatological grafting procedures to treat burns, bone defects, and skin wounds. Other factors, including extensive research and development activities in medical sciences and improving healthcare infrastructure, are also predicted to drive the market even further.
According to the Alliance for Regenerative Medicine, there are approaching approximately 1,000 companies focusing on this evolving area worldwide. These new companies are focusing on gene therapy, cell therapy and tissue engineering therapeutic developers. More than half of these companies are in North America, followed by almost a quarter in Europe and Israel and approximately 20% in Asia. More than 50% of these companies are focusing on cell therapy and gene therapy.
From 2014 to 2019, the global regenerative medicine market experienced a nearly 16% CAGR. Companies involved in gene and cell therapies as well as other regenerative medicine areas raised $4.8 billion during the first half of 2019, including $2.6 billion in the second quarter. Meanwhile, companies in Europe and Israel saw an acceleration of fundraising, with $1.3 billion amassed in just the first half of 2019, representing a 17% increase over the same period in 2018. Project Warp Speed has attracted billions of dollars of U.S. government spending, and similar efforts are ongoing in China, Russia, the European Union and among other major powers. Consequently, regenerative medicine has never before benefited from such a combination of public and private investment.
Whenever the viability and quality of human life are at stake, ethical and legal considerations always arise.
The modern ethical controversy surrounding regenerative medicine began in 1998 when research scientists at the University of Wisconsin succeeded in deriving and growing stem cells from early-stage human embryos. Ethicists and right-to-life activists protested that scientists were taking away human life (embryos) to conduct scientific experiments. Left unchecked, so the argument went, doctors could usurp nature and play God by developing the power to create and terminate life. A society where human life could be fundamentally perverted by medicine conjured up comparisons to Nazi Germany and Frankenstein. In 2001, then-U.S. President George W. Bush cut off federal funding for any research involving newly created embryonic stem cell lines, but agreed to continue funding research on 60 existing stem cell lines, where the life and death decision ha[d] already been made. The State of California responded in 2004 and again in 2020 with voter-approved programs directing billions of funding into stem cell research, making the region the global hub of regenerative medicine.
The use of human-derived embryonic stem cells, or animal-derived stem cells, continues to cause much controversy among ethicists and society at large. Some fear the risks of enrolling humans in experimental stem cell studies. Others fear the use of organs from human-animal chimeras in transplantation.
While these techniques have the potential to cure disease and save lives, they also have the potential to forever alter the nature of life as we know it and fundamental aspects of our society.
In the United States, legal jurisdiction for regulating regenerative medicine on a federal level lies with the FDA and in a patchwork of state laws, R&D funding programs and non-binding, NGO-promulgated statements of policy. The main responsibility of the FDA is to protect the public from dangerous products and ensure its safety, including overseeing medications for humans and animals, vaccines, and more.
During the Trump Administration, the FDA has largely focused on enabling developers to gain product approvals through a less burdensome and costly process. In numerous policy statements, the FDA under President Trump has deferred questions about the efficacy of new regenerative health products to the free markets, so long as they posed no serious safety or toxicity concerns.
The U.S. federal government is now transitioning to an administration led by President-elect Biden. The president-elect has spent many years advocating for increased R&D funding and going for moonshots. With a new mandate from the U.S. electorate to address the coronavirus, more money will be earmarked for regenerative medicines and stem cell research. How this will affect the release of new products into the market remains to be seen.
Regenerative medicine is poised to change the way we live, work and interact like never before. The fourth industrial revolution is upon us. CRISPR gene-editing technologies, facilitated by quantum-computing capabilities at the edge of a computer network powered by 5G telecommunications bandwidths, artificial intelligence and machine learning, have changed the game for regenerative medicine. We can foresee a day when those suffering from paralysis regain movement, when a damaged heart reverses course through regeneration, and when a diagnosis of Alzheimers Disease no longer means neurodegeneration. What a wonderful day that will be.
Changing the traditional healthcare model and moving from cure to prevention will take time.
The rise in chronic disease and the effort to reduce healthcare costs presents a large opportunity for the field of regenerative medicine.
As the continent becomes a bigger player, western companies should explore the potential prospects.
Topics from regenerative medicine to artificial intelligence to cannabis will be discussed.
The rest is here:
Regenerative Medicine: Market Trends and Legal Developments on the Horizon for 2021 - MedTech Intelligence
New health researchers at Dal, IWK and Nova Scotia Health receive funding from Research Nova Scotia – Dal News
By daniellenierenberg
Researchers with affiliations to Dalhousie University, Nova Scotia Health and the IWK Health Centre are the recipients of over $1.3 million in funding from Research Nova Scotia.
The funding has been provided by the New Health Investigator Grant, which supports new health researchers who are engaged in work that aligns with the provinces health research priorities. The grant aims to provide two years of support of up to $100,000 for researchers who are within the first five years of their academic appointment in Nova Scotia, or who are new to the field of health research.
There has never been a greater need to support new health researchers in Nova Scotia to help inform practice, policy and decision making, says Stefan Leslie, CEO of Research Nova Scotia in a news release. Were pleased to announce funding for these researchers and are confident their work will positively impact the health of Nova Scotians.
For the 2020-21 academic year, funding for this grant is provided by the Nova Scotia Department of Health and Wellness. It will support the establishment of independent programs of research and support and expand the research productivity necessary for obtaining long-term funding from national and external agencies and provide opportunities for early-career investigators to make significant contributions in their field.
Congratulations to all the recipients of funding from Research Nova Scotia, says Dr. Alice Aiken, vice president research and innovation at Dalhousie. With projects that span a wide range of topics, like diabetes, cancer, dementia care, and the COVID-19 pandemic, these researchers are improving health care and helping people in the Maritimes and beyond to be healthier.
Highlights of some of the funded projects:
Dr. Christine Cassidy, Faculty of Health
Designing an integrated pediatric inpatient-ambulatory care service delivery model
The health care system is facing challenges related to poor quality of care, rising health care costs, and outdated technology. Efforts are needed to redesign health services to improve outcomes for patients, health care providers, and the overall health system. One way to address these challenges is to integrate care across multiple health care providers and services. This means that care is coordinated to meet patient needs and preferences.
During the COVID-19 pandemic, the IWK Health Care Centre identified gaps in their current approach to delivering services to children, youth, and their families which includes the need to improve the integration of care across their outpatient and inpatient settings. Healthcare interventions are more effective when patients and care providers are included in the design process, and the integrated approach developed by Dr. Cassidy and her research team will help strengthen the delivery of care within the pediatric health system.
Dr. Parisa Ghanouni, Faculty of Health
Community-based services for individuals with developmental disabilities: Transition to adult care
Despite the great progress signaled by the United Nations Convention on the Rights of Persons with Disabilities, individuals with disabilities worldwide continue to confront barriers to equitable access to the health resources and social supports that enable their full participation in society. Gaps in access have improved for many, especially for children, but the transition to adulthood continues to represent a services cliff that people with disabilities confront in their late teens.
Through their research, Dr. Ghanouni and her team plan to uncover barriers and facilitators related to community-based healthcare services during the transition of adolescents with developmental disabilities to adulthood in rural areas, and co-develop a toolkit with stakeholders that outlines implementation strategies to promote successful transitions. This initiative will advance knowledge on services available that support the transition to adulthood in rural areas, highlight service gaps, point to important areas for investment, and contribute to academic, policy and community understandings and capacity around services for people with disabilities.
Dr. Brendan Leung, Faculty of Dentistry
Harnessing oral microbiota to prevent chemotherapy-induced oral mucositis: Functional screening using a bio-printed mammalian-microbe co-culture model
Chemotherapy induced oral mucositis (CIOM) is a painful and debilitating side effect of cancer treatment that affects 20-40% of cancer patients. Chemotherapy kills cancer cells, but it also affects fast growing normal cells in the body, especially those that line the mouth. When those are damaged, painful mouth ulcers form. These can affect patients ability to eat, drink, talk and even rest, and significantly reduce their quality of life. Currently there is no effective way to prevent CIOM from happening, and the only way to treat it is to provide supportive care such as numbing gels, ice chips and painkillers.
Research has found that the types of bacteria that normally live in the mouth change when someone develops CIOM. It is difficult to study cause and effect between bacteria and CIOM, partly because it is difficult to grow bacteria and human cells together in the lab in a controlled and repeatable way. Through his research, Dr. Leung will use a unique method to grow oral bacteria to investigate how microbes interact with oral cells during chemotherapy in order to identify microbial species that may offer protection against CIOM.
Dr. Elaine Moody, Faculty of Health
Primary healthcare for people with dementia: Exploring care provided by collaborative family practice teams in Nova Scotia
There is an increasing need to improve the health care of people with dementia in Nova Scotia. As the population ages, it will become even more important to provide good care to people with dementia to ensure they can live well in the community. In Nova Scotia, there has been a move to develop collaborative family practice teams, where physicians, nurse practitioners, family practice nurses and other healthcare providers work together to address the primary health care needs of individuals. Primary care providers in these teams require dementia-specific knowledge, skills, resources and supports to enable people with dementia and their caregivers to live well in the community.
Dr. Moody and her research team hope to better understand how collaborative family practice teams in Nova Scotia are addressing the needs of people living with dementia in the community, and to identify ways to improve their care. To achieve their goal, the researchers will gather the perspectives of people living with dementia and caregivers on how collaborative family practice teams provide care in order to identify gaps in current service provision and opportunities to improve care, with a particular focus on diversity and inclusion. Additionally, they will explore how care provided by collaborative family practice teams to people with dementia has been affected by the COVID-19 outbreak.
Other funded projects include:
Dr. Leah Cahill, Faculty of Medicine
Does a simple blood test predict who needs strict blood sugar control to prevent heart disease?
Dr. Sylvain Charlebois, Faculty of ManagementHome food gardening in response to the COVID-19 pandemic: Lessons for food security considerations
Dr. Ketul Chaudhary, Faculty of MedicineCardiac Vascular Stem Cells in Right Heart Failure
Dr. Jon Dorling, Faculty of MedicinePreterm Infant Gut microbiome associations with Environment and Outcomes in the NICU (PIGEON)
Dr. Denys Khaperskyy, Faculty of MedicineRole of stress granule formation in immune responses to respiratory viruses
Dr. Michael Kucharczyk, Faculty of MedicineCan Magnetic Resonance Imaging of the prostate combined with a Radiomics Evaluation determine the invasive capacity of a tumour (Can MRI-PREDICT)
Dr. Paula McLaughlin, Faculty of MedicineIdentifying, understanding, and mitigating gaps in dementia care
Dr. Sandra Meier, Faculty of MedicineAn app responding to behaviour of people to promote mental wellbeing in anxious youth
Dr. Deniz Top, Faculty of MedicineDifference in the regulation of behaviour genes as a proposed mechanism for mental illness
Dr. Igor Yakovenko, Faculty of ScienceScreening, self-management and referral to treatment for young cannabis users: Fulfilling an unmet need
For a complete list of recipients and projects, visit the Research Nova Scotia website.
See the original post here:
New health researchers at Dal, IWK and Nova Scotia Health receive funding from Research Nova Scotia - Dal News
BioCryst to Present at JMP Securities Hematology Summit
By Dr. Matthew Watson
RESEARCH TRIANGLE PARK, N.C., Dec. 11, 2020 (GLOBE NEWSWIRE) -- BioCryst Pharmaceuticals, Inc. (Nasdaq: BCRX) today announced that the company will present at the JMP Securities Hematology Summit, which is being conducted as a virtual event, on Tuesday, December 15, 2020 at 3:30 p.m. ET.
See more here:
BioCryst to Present at JMP Securities Hematology Summit
Altimmune to Present at the 4th Annual NASH Summit 2020 Digital Conference
By Dr. Matthew Watson
GAITHERSBURG, Md., Dec. 11, 2020 (GLOBE NEWSWIRE) -- Altimmune, Inc. (Nasdaq: ALT), a clinical-stage biopharmaceutical company, today announced that Dr. Scott Harris, Chief Medical Officer of Altimmune will give an oral presentation on ALT-801, the Company’s novel GLP-1/Glucagon dual receptor agonist in development for the treatment of non-alcoholic steatohepatitis (NASH.) Dr. Harris will also participate on a panel discussion on NASH treatments. The presentations will take place at the 4th Annual NASH Summit 2020 Digital Conference, which is being held December 15-18, 2020.
Read more here:
Altimmune to Present at the 4th Annual NASH Summit 2020 Digital Conference
New Study Provides Personalized Breast Cancer Risk Information for Women with ATM Gene Mutations
By Dr. Matthew Watson
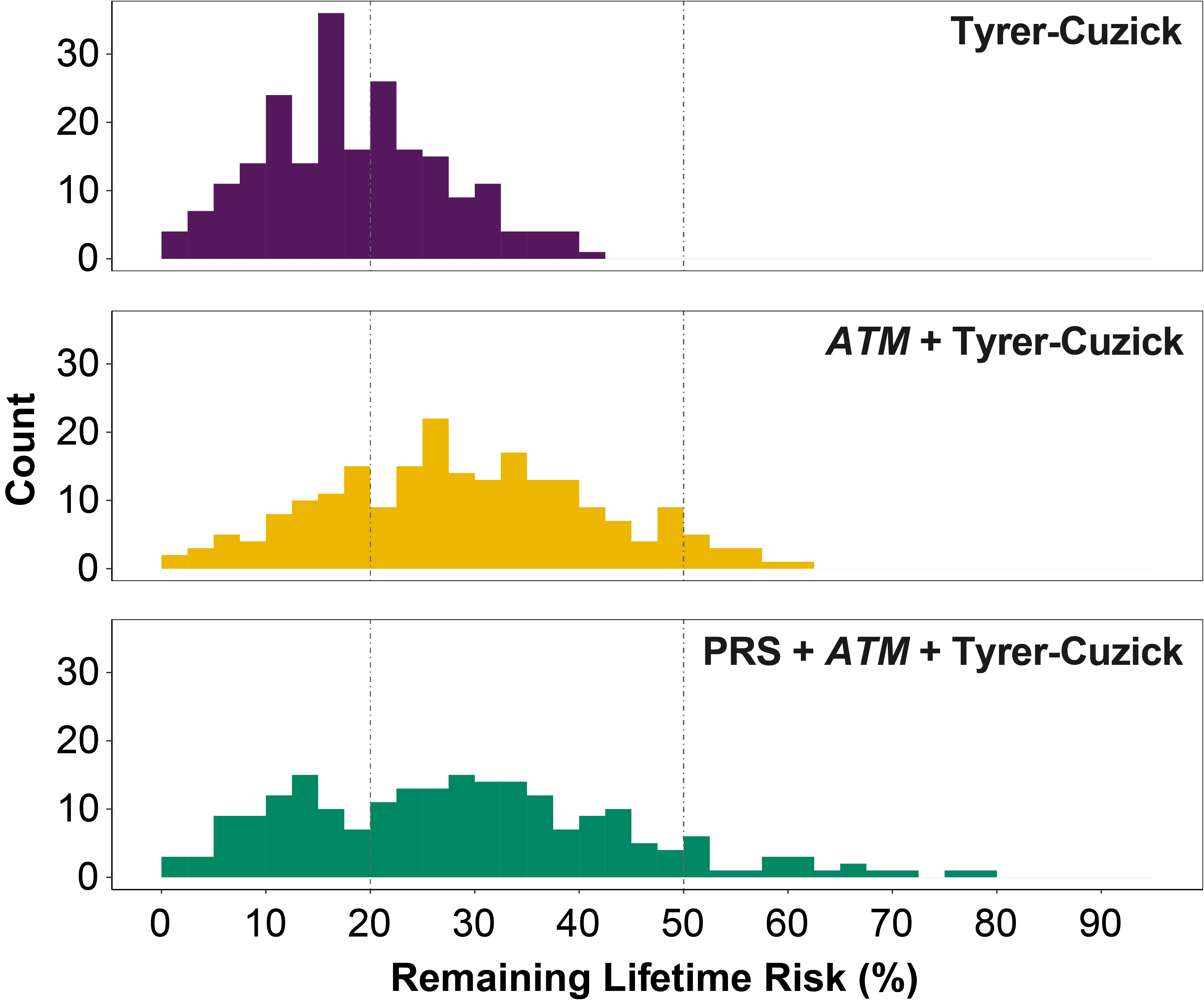
Here is the original post:
New Study Provides Personalized Breast Cancer Risk Information for Women with ATM Gene Mutations
Lexicon Pharmaceuticals Receives Fast Track Designation From the FDA for LX9211 for Diabetic Peripheral Neuropathic Pain
By Dr. Matthew Watson
THE WOODLANDS, Texas, Dec. 11, 2020 (GLOBE NEWSWIRE) -- Lexicon Pharmaceuticals, Inc. (Nasdaq: LXRX), announced today that it has received Fast Track designation from the U.S. Food and Drug Administration (FDA) for the development of LX9211 in diabetic peripheral neuropathic pain.
See the rest here:
Lexicon Pharmaceuticals Receives Fast Track Designation From the FDA for LX9211 for Diabetic Peripheral Neuropathic Pain
Arch Therapeutics to Present at the 13th Annual LD Micro Main Event Conference
By Dr. Matthew Watson
FRAMINGHAM, Mass., Dec. 11, 2020 (GLOBE NEWSWIRE) -- Arch Therapeutics, Inc. (OTCQB: ARTH) ("Arch" or the "Company"), developer of novel self-assembling wound care and biosurgical devices, announced today that it will be presenting at the 13th annual LD Micro Main Event investor conference. This investor conference will take place December 14-15, 2020, on the Sequire Virtual Events platform.
See original here:
Arch Therapeutics to Present at the 13th Annual LD Micro Main Event Conference
ExCellThera receives Priority Medicines (PRIME) designation from European Medicines Agency for ECT-001 Cell Therapy
By Dr. Matthew Watson
MONTREAL, Dec. 11, 2020 (GLOBE NEWSWIRE) -- ExCellThera Inc., a clinical-stage molecular medicine company delivering molecules and bioengineering solutions to expand stem and immune cells for therapeutic use, announced today that ECT-001 Cell Therapy has been granted PRIority MEdicines (PRIME) designation by the European Medicines Agency (EMA) for use in urgent allogeneic haematopoietic stem cell transplants.
The rest is here:
ExCellThera receives Priority Medicines (PRIME) designation from European Medicines Agency for ECT-001 Cell Therapy


 December 13th, 2020
December 13th, 2020